Difference between revisions of "Expt:Preparing biodiesel"
m (Fix cat) |
m (Add link) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | This is an online version of the experiment. This procedure is also available in [http://www.soton.ac.uk/chemistry/schoolsoutreach/A-level/Preparing%20biodiesel%20-%20Student.doc Microsoft Word] and PDF formats. | + | {{ClimateChangeExpts}} |
| + | This is an online version of the experiment '''[[Lab:Preparing biodiesel|"Preparing biodiesel"]]'''.. This procedure is also available in [http://www.soton.ac.uk/chemistry/schoolsoutreach/A-level/Preparing%20biodiesel%20-%20Student.doc Microsoft Word] and '''[[:File:Preparing biodiesel - Student.pdf|PDF]]''' formats. There are also '''[[TeacherExpt:Preparing biodiesel|teacher and technician notes]]''' for this experiment. | ||
==Activity== | ==Activity== | ||
| Line 37: | Line 38: | ||
d. How could you compare the effectiveness of biodiesel as a fuel with that of regular ‘fossil’ diesel?<br/> | d. How could you compare the effectiveness of biodiesel as a fuel with that of regular ‘fossil’ diesel?<br/> | ||
e. How could you evaluate the environmental benefits of using biodiesel? (Hint: it’s not just about carbon dioxide!).<br/> | e. How could you evaluate the environmental benefits of using biodiesel? (Hint: it’s not just about carbon dioxide!).<br/> | ||
| + | |||
| + | ==Source== | ||
| + | Experiment written by David Read (School Teacher Fellow, [http://www.soton.ac.uk/chemistry/ School of Chemistry, University of Southampton]), adapted from a method found at '''''[http://www.greeningschools.org www.greeningschools.org]'''''. | ||
[[Category:ChemSpider Education]] | [[Category:ChemSpider Education]] | ||
| − | [[Category: | + | [[Category:Biofuels]] |
[[Category:University of Southampton resources]] | [[Category:University of Southampton resources]] | ||
Latest revision as of 01:24, 19 August 2010
| Experiments related to climate change |
|---|
| Experiments on: |
| For teachers |
| For students |
| For technicians |
This is an online version of the experiment "Preparing biodiesel".. This procedure is also available in Microsoft Word and PDF formats. There are also teacher and technician notes for this experiment.
Contents
Activity
You will prepare a small sample of biodiesel by reacting vegetable oil with methanol in the presence of a catalyst.
Background
The use of biodiesel as a fuel has taken off over the last couple of years. Initially, biofuels were viewed as a fool-proof solution to the impending energy crisis. Biofuels are considered by many to be carbon neutral since the plants they are made from grow by taking in carbon dioxide from the air. Hence the carbon dioxide emitted by a vehicle running on a biofuel will then be reabsorbed by the plants in the next crop. It’s a nice idea in theory, and consumers and governments alike have bought into the biofuel revolution.
In reality, there is an emerging debate about the true benefits of biofuels, and many people dispute that they really are carbon neutral. However, biofuels do have the potential to help bridge the gap as we wean ourselves off fossil fuels, and there is a lot of research into more sustainable methods of producing them (so-called ‘second generation biofuels’).
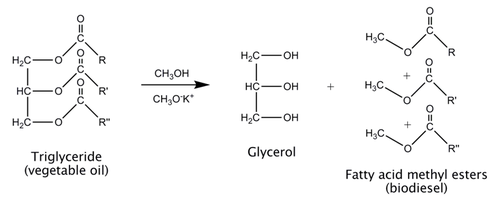
Biodiesel is commonly made using a process called transesterification. Vegetable oil is composed of triglycerides, which are triesters of glycerol. To make biodiesel, the triglycerides are reacted with methanol. The reaction is extremely slow, so a potassium methoxide catalyst is used to speed the reaction up.
Note: The catalyst is made by dissolving 5 g potassium hydroxide in 100 cm3 methanol. The potassium hydroxide acts as a base, removing a proton from the methanol to make potassium methoxide.
Equipment needed
Boiling tube, boiling tube rack, bung/stopper, 10 cm3 measuring cylinder, vegetable oil, 5 % potassium methoxide in methanol.
- Safety
Wear safety glasses and apron/labcoat at all times.
- Procedure
Record all observations (colour, viscosity, anything else) at all stages of the practical.
- Measure 10 cm3 of vegetable oil and pour into a boiling tube.
- Add 1.5 cm3 of the potassium methoxide catalyst dissolved in methanol using a pipette in a fume cupboard and place the bung/stopper in the tube.
- Invert the tube repeatedly over a period of 10 minutes without shaking.
- Pause periodically and observe any changes that occur in the properties of the contents of the tube.
- After 10 minutes of mixing, place the tube in a test-tube rack and let the contents settle. You should see two layers. You may need to leave it overnight for the layers to separate completely.
- The top layer is biodiesel, which is floating on top of the glycerol by-product.
- Questions
a. What changes did you observe in the properties of the contents of the tube as the reaction proceeded?
b. What are the differences between the properties of vegetable oil and biodiesel that make the latter more suitable as a fuel?
c. What do you think has happened to the amount of glycerol produced worldwide in recent years? What are the economic consequences of this? How do you think chemists can capitalise on the situation?
d. How could you compare the effectiveness of biodiesel as a fuel with that of regular ‘fossil’ diesel?
e. How could you evaluate the environmental benefits of using biodiesel? (Hint: it’s not just about carbon dioxide!).
Source
Experiment written by David Read (School Teacher Fellow, School of Chemistry, University of Southampton), adapted from a method found at www.greeningschools.org.