Difference between revisions of "Schlenk line"
Physchim62 (talk | contribs) (Reimported from http://en.wikipedia.org/w/index.php?title=Schlenk_line&oldid=298743639 for copyright reasons) |
Physchim62 (talk | contribs) |
||
| Line 40: | Line 40: | ||
* {{cite web | author = Rob Toreki | title = Schlenk Lines and Vacuum Lines | url = http://www.ilpi.com/inorganic/glassware/vacline.html | work = The Glassware Gallery | publisher = Interactive Learning Paradigms Incorporated | date = 25 May 2004}} | * {{cite web | author = Rob Toreki | title = Schlenk Lines and Vacuum Lines | url = http://www.ilpi.com/inorganic/glassware/vacline.html | work = The Glassware Gallery | publisher = Interactive Learning Paradigms Incorporated | date = 25 May 2004}} | ||
| − | |||
[[Category:Laboratory glassware]] | [[Category:Laboratory glassware]] | ||
[[Category:Air-free techniques]] | [[Category:Air-free techniques]] | ||
{{Imported from Wikipedia|name=Schlenk line|id=298743639}} | {{Imported from Wikipedia|name=Schlenk line|id=298743639}} | ||
Latest revision as of 16:29, 9 August 2009
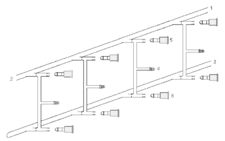
The Schlenk line (also vacuum gas manifold) is a commonly-used piece of chemistry apparatus developed by Wilhelm Schlenk. It consists of a dual manifold with several ports.[1] One manifold is connected to a source of purified inert gas, while the other is connected to a high-vacuum pump. The inert gas line is vented through an oil bubbler, while solvent vapors and gaseous reaction products are prevented from contaminating the pump through a liquid nitrogen or dry ice/acetone cold trap. Special stopcocks or Teflon taps allow for vacuum or inert gas to be selected without the need for placing the sample on a separate line.
Schlenk lines are useful for safely and successfully manipulating air sensitive compounds. The high vacuum is also often used to remove the last traces of solvent from a sample. Vacuum gas manifolds often have many ports and lines, and with care it is possible for several reactions or operations to be run simultaneously.
Techniques
The main techniques associated with the use of a Schlenk line include:
- counterflow additions, where air-stable reagents are added to the reaction vessel against a flow of inert gas.
- the use of syringes and rubber septa to transfer liquids and solutions
- cannula transfer, where liquids or solutions of air-sensitive reagents are transferred between different vessels stoppered with septa using a long thin tube known as a cannula. Liquid flow is achieved via vacuum or inert gas pressure.[2]
 A cannula is used to transfer THF from the flask on the right to the flask on the left.
A cannula is used to transfer THF from the flask on the right to the flask on the left.
Glassware are usually connected via tightly-fitting and greased ground glass joints. Round bends of glass tubing with ground glass joints may be used to adjust the orientation of various vessels.
Filtration under inert conditions poses a special challenge that is usually tackled with specialized glassware. A Schlenk filter consists of sintered glass funnel fitted with joints and stopcocks. By fitting the pre-dried funnel and receiving flask to the reaction flask against a flow of nitrogen, carefully inverting the set-up, and turning on the vacuum appropriately, the filtration may be accomplished with minimal exposure to air.
Dangers
The main dangers associated with the use of a Schlenk line are the risks of an implosion or explosion. An implosion can occur due to the use of a high vacuum and flaws in the glass apparatus.
An explosion can occur due to the common use of liquid nitrogen in the cold trap, used to protect the vacuum pump from solvents. If a reasonable amount of air is allowed to enter the Schlenk line, liquid oxygen can condense into the cold trap as a pale blue liquid. An explosion may occur due to reaction of the liquid oxygen with any organic compounds also in the trap.
See also
- Air-free technique gives a broad overview of methods including
- Glovebox - used to manipulate air-sensitive (oxygen- or moisture-sensitive) chemicals.
- Schlenk flask - reaction vessel for handling air-sensitive compounds.
- Perkin triangle - used for the distillation of air-sensitive compounds.
References
- ↑ Craig M. Davis and Kelly A. Curran (November 2007). "Manipulation of a Schlenk Line: Preparation of Tetrahydrofuran Complexes of Transition-Metal Chlorides" (abstract page). Journal of Chemical Education 84 (11): 1822–3.
- ↑ Brown, H. C. “Organic Syntheses via Boranes” John Wiley & Sons, Inc. New York: 1975. ISBN 0-471-11280-1.
Further reading
- Sella, Andrea (January 2008). "Schlenk Apparatus". Chemistry World: 69. Retrieved on 2008-01-30.
- Tidwell, Thomas (2001). "Wilhelm Schlenk: The Man Behind the Flask". Angewandte Chemie International Edition 40: 331–337. doi:<331::AID-ANIE331>3.0.CO;2-E 10.1002/1521-3773(20010119)40:2<331::AID-ANIE331>3.0.CO;2-E.
- Jürgen Heck. The Integrated Synthesis Course: Schlenk Technique (reprint at Norwegian University of Science and Technology). University of Hamburg.
- Handling and Storage of Air-Sensitive Reagents (PDF). Sigma-Aldrich.
External links
- Rob Toreki (25 May 2004). Schlenk Lines and Vacuum Lines. The Glassware Gallery. Interactive Learning Paradigms Incorporated.
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Schlenk line". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |