Difference between revisions of "Sarsasapogenin"
Physchim62 (talk | contribs) |
Physchim62 (talk | contribs) |
||
| Line 23: | Line 23: | ||
Sarsasapogenin is unusual in that it has a ''cis''-linkage between rings A and B of the steroid nucleus, as opposed to the more usual ''trans''-linkage found in other saturated steroids. This 5β configuration is biologically significant, as a specific enzyme – [[sarsasapogenin 3β-glucosyltransferase]] – is found in several plants for the glycosylation of sarsasapogenin.<ref>{{citation | last1 = Paczkowski | first1 = Cezary | last2 = Wojciechowski | first2 = Zdzisław A. | title = The occurrence of UDPG-dependent glucosyltransferase specific for sarsasapogenin in ''Asparagus officinalis'' | journal = Phytochemistry | volume = 27 | issue = 9 | year = 1988 | pages = 2743–47 | doi = 10.1016/0031-9422(88)80654-X}}.</ref> The (''S'')-configuration at C-25 is also in contrast to other spirostan sapogenins: the epimer with a (25''R'')-configuration is known as [[smilagenin]]. | Sarsasapogenin is unusual in that it has a ''cis''-linkage between rings A and B of the steroid nucleus, as opposed to the more usual ''trans''-linkage found in other saturated steroids. This 5β configuration is biologically significant, as a specific enzyme – [[sarsasapogenin 3β-glucosyltransferase]] – is found in several plants for the glycosylation of sarsasapogenin.<ref>{{citation | last1 = Paczkowski | first1 = Cezary | last2 = Wojciechowski | first2 = Zdzisław A. | title = The occurrence of UDPG-dependent glucosyltransferase specific for sarsasapogenin in ''Asparagus officinalis'' | journal = Phytochemistry | volume = 27 | issue = 9 | year = 1988 | pages = 2743–47 | doi = 10.1016/0031-9422(88)80654-X}}.</ref> The (''S'')-configuration at C-25 is also in contrast to other spirostan sapogenins: the epimer with a (25''R'')-configuration is known as [[smilagenin]]. | ||
| − | Sarsasapogenin has been used as a starting material for the synthesis of other steroids.<ref>{{citation | inventor1-last = Dryden, Jr. | inventor1-first = Hugh L. | inventor2-last = Markos | inventor2-first = Charles S. | assignee = Searle & Co. | country-code = US | patent-number = 4057543 | publication-date = 1977-11-08}}.</ref> It has also attracted pharmaceutical interest in its own right,<ref>{{citation | inventor1-last = Applezweig | inventor1-first = Norman | assignee = Progenics Inc. | country-code = US | patent-number = 4680289 | publication-date = 1987-07-14}}.</ref><ref>{{citation | inventor1-last = Xia | inventor1-first = Zongqin | inventor2-last = Hu | inventor2-first = Yaer | inventor3-last = Rubin | inventor3-first = Ian | inventor4-last = Brostoff | inventor4-first = Jonathan | inventor5-last = Whittle | inventor5-first = Brian | inventor6-last = Wang | inventor6-first = Weijun | inventor7-last = Gunning | inventor7-first = Phil | assignee = Phytopharm plc | title = Steroidal sapogenins and their derivatives for treating alzheimer's disease | country-code = US | patent-number = 6812213 | publication-date = 2002-12-19 }}.</ref> and is found in the rhizome of ''Anemarrhena asphodeloides'', used in Chinese tradition medicine (知母, ''zhī mǔ''), from which it is extracted commercially.<ref name="Wilshire">{{citation | url = http://www.wilshiretechnologies.com/steroidal_saponins_and_sapogenins_pdf/sarsasapogenin.pdf | title = Sarsasapogenin | publisher = Wilshire Technologies | accessdate = 2010-03-07}}.</ref> | + | Sarsasapogenin has been used as a starting material for the synthesis of other steroids.<ref>{{citation | inventor1-last = Dryden, Jr. | inventor1-first = Hugh L. | inventor2-last = Markos | inventor2-first = Charles S. | assignee = Searle & Co. | title = Process for the preparation of 17β-hydroxy-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone | country-code = US | patent-number = 4057543 | publication-date = 1977-11-08}}.</ref> It has also attracted pharmaceutical interest in its own right,<ref>{{citation | inventor1-last = Applezweig | inventor1-first = Norman | assignee = Progenics Inc. | title = Treatment of obesity and diabetes using sapogenins | country-code = US | patent-number = 4680289 | publication-date = 1987-07-14}}.</ref><ref>{{citation | inventor1-last = Xia | inventor1-first = Zongqin | inventor2-last = Hu | inventor2-first = Yaer | inventor3-last = Rubin | inventor3-first = Ian | inventor4-last = Brostoff | inventor4-first = Jonathan | inventor5-last = Whittle | inventor5-first = Brian | inventor6-last = Wang | inventor6-first = Weijun | inventor7-last = Gunning | inventor7-first = Phil | assignee = Phytopharm plc | title = Steroidal sapogenins and their derivatives for treating alzheimer's disease | country-code = US | patent-number = 6812213 | publication-date = 2002-12-19}}.</ref> and is found in the rhizome of ''Anemarrhena asphodeloides'', used in Chinese tradition medicine (知母, ''zhī mǔ''), from which it is extracted commercially.<ref name="Wilshire">{{citation | url = http://www.wilshiretechnologies.com/steroidal_saponins_and_sapogenins_pdf/sarsasapogenin.pdf | title = Sarsasapogenin | publisher = Wilshire Technologies | accessdate = 2010-03-07}}.</ref> |
==References== | ==References== | ||
Revision as of 13:55, 7 March 2010
| Sarsasapogenin | |
|---|---|
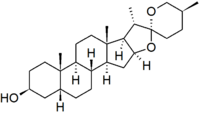
| |
| IUPAC name | (3β,5β,25S)-spirostan-3-ol |
| Identifiers | |
| InChI | InChI=1/C27H44O3/c1-16-7-12-27 (29-15-16)17(2)24-23(30-27)14- 22-20-6-5-18-13-19(28)8-10-25( 18,3)21(20)9-11-26(22,24)4/h16 -24,28H,5-15H2,1-4H3/t16-,17-, 18+,19-,20+,21-,22-,23-,24-,25 -,26-,27+/m0/s1 |
| InChIKey | GMBQZIIUCVWOCD-WWASVFFGBR |
| Standard InChI | InChI=1S/C27H44O3/c1-16-7-12-2 7(29-15-16)17(2)24-23(30-27)14 -22-20-6-5-18-13-19(28)8-10-25 (18,3)21(20)9-11-26(22,24)4/h1 6-24,28H,5-15H2,1-4H3/t16-,17- ,18+,19-,20+,21-,22-,23-,24-,2 5-,26-,27+/m0/s1 |
| Standard InChIKey | GMBQZIIUCVWOCD-WWASVFFGSA-N |
| CAS number | [] |
| EC number | |
| ChemSpider | |
| Properties[1] | |
| Chemical formula | C27H44O3 |
| Molar mass | 416.64 g/mol |
| Melting point |
199–199.5 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Sarsasapogenin is a steroidal sapogenin, that is the aglycosidic portion of a plant saponin. It is named after sarsaparilla (Smilax sp.),[2] a family of climbing plants found in subtropical regions. It was one of the first sapogenins to be identified,[2] and the first spirostan steroid to be identified as such.[3] The identification of the spirostan structure, with its ketone spiro acetal functionality, was fundamental in the development of the Marker degradation, which allowed the industrial production of progesterone and other sex hormones from plant steroids.
Sarsasapogenin is unusual in that it has a cis-linkage between rings A and B of the steroid nucleus, as opposed to the more usual trans-linkage found in other saturated steroids. This 5β configuration is biologically significant, as a specific enzyme – sarsasapogenin 3β-glucosyltransferase – is found in several plants for the glycosylation of sarsasapogenin.[4] The (S)-configuration at C-25 is also in contrast to other spirostan sapogenins: the epimer with a (25R)-configuration is known as smilagenin.
Sarsasapogenin has been used as a starting material for the synthesis of other steroids.[5] It has also attracted pharmaceutical interest in its own right,[6][7] and is found in the rhizome of Anemarrhena asphodeloides, used in Chinese tradition medicine (知母, zhī mǔ), from which it is extracted commercially.[8]
References
- ↑ Jacobs, Walter A.; Simpson, James C. E. On Sarsasapogenin and Gitogenin. J. Biol. Chem. 1934, 105 (3), 501–10, <http://www.jbc.org/content/105/3/501.full.pdf>.
- ↑ 2.0 2.1 Power, Frederick Belding; Salway, Arthur Henry Chemical examination of sarsaparilla root. J. Chem. Soc., Trans. 1914, 105, 201–19. DOI: 10.1039/CT9140500201.
- ↑ Marker, Russell E.; Rohrmann, Ewald Sterols. LIII. The Structure of the Side Chain of Sarsasapogenin. J. Am. Chem. Soc. 1939, 61 (4), 846–51. DOI: 10.1021/ja01873a020.
- ↑ Paczkowski, Cezary; Wojciechowski, Zdzisław A. The occurrence of UDPG-dependent glucosyltransferase specific for sarsasapogenin in Asparagus officinalis. Phytochemistry 1988, 27 (9), 2743–47. DOI: 10.1016/0031-9422(88)80654-X.
- ↑ Dryden, Jr., Hugh L.; Markos, Charles S. (Searle & Co.) Process for the preparation of 17β-hydroxy-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone. US Patent 4057543, published 8 November 1977.
- ↑ Applezweig, Norman (Progenics Inc.) Treatment of obesity and diabetes using sapogenins. US Patent 4680289, published 14 July 1987.
- ↑ Xia, Zongqin; Hu, Yaer; Rubin, Ian, et al. (Phytopharm plc) Steroidal sapogenins and their derivatives for treating alzheimer's disease. US Patent 6812213, published 19 December 2002.
- ↑ Sarsasapogenin; Wilshire Technologies, <http://www.wilshiretechnologies.com/steroidal_saponins_and_sapogenins_pdf/sarsasapogenin.pdf>. (accessed 7 March 2010).
| Error creating thumbnail: Unable to save thumbnail to destination |
This page is currently licensed under the Creative Commons Attribution 3.0 Unported license and any later versions of that license. |