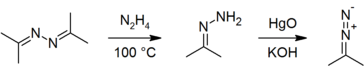Difference between revisions of "Azine"
Physchim62 (talk | contribs) |
Physchim62 (talk | contribs) (→Reactions and uses) |
||
| (17 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | '''Azines''' are a [[functional class]] of [[organic compound]]s, formed from the reaction of two [[equivalent (chemistry)|equivalent]]s of an [[aldehyde]] or [[ketone]] with one equivalent of [[hydrazine]]. They may be further classified as '''aldazines''' or '''ketazines''', depending on the nature of the [[carbonyl compound]]. | + | {{about|the hydrazine derivatives|the use of "azine" in the names of heterocyclic compounds|Hantzsch–Widman nomenclature}} |
| + | [[File:Azine.png|thumb|right|The generic formula of an azine. For an aldazine, R<sup>2</sup> = H.]] | ||
| + | '''Azines''' are a [[functional class]] of [[organic compound]]s, formed from the [[condensation reaction]] of two [[equivalent (chemistry)|equivalent]]s of an [[aldehyde]] or [[ketone]] with one equivalent of [[hydrazine]].<ref>{{IUPAC class names 1995|page=1321}}.</ref><ref>{{GoldBookRef|title=azines|file=A00557|accessdate=2010-07-02}}.</ref> They may be further classified as '''aldazines''' or '''ketazines''', depending on the nature of the [[carbonyl compound]].<ref>{{IUPAC class names 1995|pages=1312, 1348}}.</ref><ref>{{GoldBookRef|title=aldazines|file=A00207|accessdate=2010-07-02}}. {{GoldBookRef|title=ketazines|file=K03377|accessdate=2010-07-02}}.</ref> | ||
| + | |||
| + | ==Preparation== | ||
| + | Azines may be prepared by the direct reaction of a carbonyl compound with [[hydrazine hydrate]]:<ref name="OrgSynth">{{OrgSynth | first1 = A. C. | last1 = Day | first2 = M. C. | last2 = Whiting | title = Acetone hydrazone | collvol = 6 | collvolpages = 10 | volume = 50 | pages =3 | year = 1970 | prep = cv6p0010}}.</ref> the reaction is exothermic.<ref>{{citation | first = E. C. | last = Gilbert | title = Studies on Hydrazine. The Hydrolysis of Dimethylketazine and the Equilibrium between Hydrazine and Acetone | journal = J. Am. Chem. Soc. | year = 1929 | volume = 51 | issue = 11 | pages = 3394–3409 | doi = 10.1021/ja01386a032}}.</ref> The usual method of industrial production is the [[Pechiney-Ugine-Kuhlmann process]], starting from the ketone, [[ammonia]] and [[hydrogen peroxide]].<ref name="PCUK">{{citation | inventor1-first = Jean-Pierre | inventor1-last = Schirmann | inventor2-first = Jean | inventor2-last = Combroux | inventor3-first = Serge Yvon | inventor3-last = Delavarenne | assignee = Produits Chimiques Ugine Kuhlmann | country-code = US | patent-number = 3972878 | title = Method for preparing azines and hydrazones | issue-date = 1976-08-03}}. {{citation | inventor1-first = Jean-Pierre | inventor1-last = Schirmann | inventor2-first = Pierre | inventor2-last = Tellier | inventor3-first = Henri | inventor3-last = Mathais | inventor4-first = Francis | inventor4-last = Weiss | assignee = Produits Chimiques Ugine Kuhlmann | country-code = US | patent-number = 3978049 | title = Process for the preparation of hydrazine compounds | issue-date = 1976-08-31}}. {{citation | inventor1-first = Jean-Pierre | inventor1-last = Schirmann | inventor2-first = Jean | inventor2-last = Combroux | inventor3-first = Serge Yvon | inventor3-last = Delavarenne | assignee = Produits Chimiques Ugine Kuhlmann | country-code = US | patent-number = 4093656 | title = Method for making azines | issue-date = 1978-06-06}}.</ref><ref name="Atochem">{{citation | inventor1-first = Jean-Pierre | inventor1-last = Schirmann | inventor2-first = Jean | inventor2-last = Combroux | inventor3-first = Serge Y. | inventor3-last = Delavarenne | assignee = Atochem | country-code = US | patent-number = 4724133 | title = Preparation of a concentrated aqueous solution of hydrazine hydrate | issue-date = 1988-02-09}}.</ref> | ||
| + | |||
| + | ==Reactions and uses== | ||
| + | Azines have been used as precursors to [[hydrazone]]s<ref name="OrgSynth"/><ref name="Ber">{{citation | first1 = H. | last1 = Staudinger | first2 = Alice | last2 = Gaule | title = Vergleich der Stickstoff-Abspaltung bei verschiedenen aliphatischen Diazoverbindungen | journal = Ber. Dtsch. Chem. Ges. | year = 1916 | volume = 49 | issue = 2 | pages = 1897–1918 | doi = 10.1002/cber.19160490245}}.</ref> and [[diazo compound]]s.<ref name="Ber"/><ref>{{citation | first1 = A. C. | last1 = Day | first2 = P. | last2 = Raymond | first3 = R. M. | last3 = Southam | first4 = M. C. | last4 = Whiting | title = The preparation of secondary aliphatic diazo-compounds from hydrazones | journal = J. Chem. Soc. C | year = 1966 | pages = 467–69 | doi = 10.1039/J39660000467}}.</ref><ref name="diazo">{{OrgSynth | first1 = S. D. | last1 = Andrews | first2 = A. C. | last2 = Day | first3 = P. | last3 = Raymond | first4 = M. C. | last4 = Whiting | title = 2-Diazopropane | collvol = 6 | collvolpages = 392 | volume = 50 | pages = 27 | year = 1970 | prep = cv6p0392}}.</ref> | ||
| + | |||
| + | [[File:Acetone azine chem.png|364px]] | ||
| + | |||
| + | Azines are also important intermediates in the industrial production of hydrazine hydrate by the [[Bayer hydrazine process]]<ref name="Bayer">{{citation | inventor1-last = Eichenhofer | inventor1-first = Kurt-Wilhelm | inventor2-last = Schliebs | inventor2-first = Reinhard | assignee = Bayer | title = Production of ketazines | country-code = US | patent-number = 3965097 | issue-date = 1976-06-22}}.</ref><ref name="H&W">{{Holleman&Wiberg|page=619}}.</ref> or the [[Pechiney-Ugine-Kuhlmann process]].<ref name="PCUK"/><ref name="Atochem"/> They have been also used as sources of hydrazine produced ''in situ'', for example in the production of [[herbicide]] precursor [[1,2,4-triazole]].<ref>{{citation | inventor1-last = Nagata | inventor1-first = Nobuhiro | inventor2-last = Nishizawa | inventor2-first = Chiharu | inventor3-last = Kurai | inventor3-first = Toshikiyo | assignee = Mitsubishi Gas Chemical Co. | title = Method of producing 1,2,4-triazole | country-code = US | patent-number = 6002015 | issue-date = 1999-12-14}}.</ref> | ||
| + | |||
| + | The [[coordination chemistry]] of azines (as [[ligand]]s) has also been studied.<ref>{{citation | first1 = A. S. | last1 = Gudkova | first2 = O. A. | last2 = Reutov | first3 = M. Ya. | last3 = Aleinikova | journal = Izv. Akad. Nauk SSSR, Otdel. Khim. Nauk | year = 1962 | issue = 8 | pages = 1382–87}}; {{citation | title = Reactions of hydrazones and azines with metal salts 4. Reactions of azines of aldehydes and ketones with cupric salts | journal = Russ. Chem. Bull. (Transl.) | year = 1962 | volume = 11 | issue = 8 | pages = 1298–1302 | doi = 10.1007/BF00907973}}.</ref><ref>{{citation | first1 = A. S. | last1 = Gudkova | first2 = M. Ya. | last2 = Aleinikova | first3 = O. A. | last3 = Reutov | journal = Izv. Akad. Nauk SSSR, Ser. Khim. | issue = 5 | pages = 844–48 | year = 1966}}; {{citation | title = Reactions of hydrazones and azines with metal salts 5. Reactions of hydrazones and azines with mercuric halides | journal = Russ. Chem. Bull. (Transl.) | volume = 15 | issue = 5 | year = 1966 | pages = 807–11 | doi = 10.1007/BF00849376}}.</ref><ref>{{citation | first1 = Fiona | last1 = King | first2 = David | last2 = Nicholls | title = Complex of titanium halides with acetone azine and its isomer 3,5,5-trimethyl-pyrazoline | journal = Inorg. Chim. Acta | volume = 28 | year = 1978 | pages = 55–58 | doi = 10.1016/S0020-1693(00)87413-7}}.</ref> [[Acetone]] is used to derivatize hydrazine, through formation of [[acetone azine]], for analysis by [[gas chromatography]]: the method has been used to determine trace levels of hydrazine in drinking water<ref>{{citation | journal = Anal. Chem. | year = 2008 | volume = 80 | issue = 14 | pages = 5449–53 | title = Analysis of hydrazine in drinking water by isotope dilution gas chromatography/tandem mass spectrometry with derivatization and liquid-liquid extraction | last1 = Davis | first1 = William E., II | last2 = Li | first2 = Yongtao | doi = 10.1021/ac702536d}}.</ref> and pharmaceuticals.<ref>{{citation | journal = J. Pharm. Biomed. Anal. | year = 2009 | volume = 49 | issue = 2 | pages = 529–33 | title = A generic approach for the determination of trace hydrazine in drug substances using ''in situ'' derivatization-headspace GC–MS | last1 = Sun | first1 = Mingjiang | last2 = Bai | first2 = Lin | last3 = Liu | first3 = David Q. | doi = 10.1016/j.jpba.2008.11.009}}.</ref> | ||
==Nomenclature== | ==Nomenclature== | ||
| − | Azines may be named by [[substitutive nomenclature|substitutive]] or [[functional class nomenclature]].<ref>{{BlueBook1993|rule= | + | Azines may be named by [[substitutive nomenclature|substitutive]] or [[functional class nomenclature]].<ref name="BB93">{{BlueBook1993|rule=5.6.6.3|page=105|url=http://www.acdlabs.com/iupac/nomenclature/93/r93_469.htm}}.</ref><ref>{{BlueBook2004|rule=68.3.1.2.3}}.</ref> In functional class nomenclature, the functional modifier "azine" is appended to the name of the carbonyl compound: hence, "[[acetone azine]]".<ref name="BB93"/> In older nomenclature, the functional class name "ketazine" has been used with the names of the hydrocarbyl substituents: e.g., "[[methyl ethyl ketazine]]". In substitutive nomenclature, azines are named as derivatives of hydrazine: hence, "[[diisopropylidenehydrazine]]".<ref name="BB93"/> In the presence of groups of higher seniority, the prefixes "hydrazinylidene" and "hydrazinediylidene" are used.<ref>{{BlueBook2004|rule=53.6.2}}.</ref> |
| + | |||
| + | Unsymmetrical azines, that is compounds of the type X=N–N=Y with X ≠ Y, are not named as azines: in the absence of other functional groups having higher seniority, they can be named as substituted [[hydrazone]]s.<ref>{{BlueBook1979|rule=C-923.2|url=http://www.acdlabs.com/iupac/nomenclature/79/r79_623.htm}}.</ref> | ||
==References== | ==References== | ||
| − | {{reflist}} | + | {{reflist|2}} |
[[Category:Azines| ]] | [[Category:Azines| ]] | ||
Latest revision as of 14:44, 3 July 2010
Azines are a functional class of organic compounds, formed from the condensation reaction of two equivalents of an aldehyde or ketone with one equivalent of hydrazine.[1][2] They may be further classified as aldazines or ketazines, depending on the nature of the carbonyl compound.[3][4]
Preparation
Azines may be prepared by the direct reaction of a carbonyl compound with hydrazine hydrate:[5] the reaction is exothermic.[6] The usual method of industrial production is the Pechiney-Ugine-Kuhlmann process, starting from the ketone, ammonia and hydrogen peroxide.[7][8]
Reactions and uses
Azines have been used as precursors to hydrazones[5][9] and diazo compounds.[9][10][11]
Azines are also important intermediates in the industrial production of hydrazine hydrate by the Bayer hydrazine process[12][13] or the Pechiney-Ugine-Kuhlmann process.[7][8] They have been also used as sources of hydrazine produced in situ, for example in the production of herbicide precursor 1,2,4-triazole.[14]
The coordination chemistry of azines (as ligands) has also been studied.[15][16][17] Acetone is used to derivatize hydrazine, through formation of acetone azine, for analysis by gas chromatography: the method has been used to determine trace levels of hydrazine in drinking water[18] and pharmaceuticals.[19]
Nomenclature
Azines may be named by substitutive or functional class nomenclature.[20][21] In functional class nomenclature, the functional modifier "azine" is appended to the name of the carbonyl compound: hence, "acetone azine".[20] In older nomenclature, the functional class name "ketazine" has been used with the names of the hydrocarbyl substituents: e.g., "methyl ethyl ketazine". In substitutive nomenclature, azines are named as derivatives of hydrazine: hence, "diisopropylidenehydrazine".[20] In the presence of groups of higher seniority, the prefixes "hydrazinylidene" and "hydrazinediylidene" are used.[22]
Unsymmetrical azines, that is compounds of the type X=N–N=Y with X ≠ Y, are not named as azines: in the absence of other functional groups having higher seniority, they can be named as substituted hydrazones.[23]
References
- ↑ Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67 (8-9), 1307–75 at 1321. DOI: 10.1351/pac199567081307.
- ↑ azines, <http://goldbook.iupac.org/A00557.html> (accessed 2 July 2010), Compendium of Chemical Terminology Internet edition; International Union of Pure and Applied Chemistry (IUPAC).
- ↑ Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67 (8-9), 1307–75 at 1312, 1348. DOI: 10.1351/pac199567081307.
- ↑ aldazines, <http://goldbook.iupac.org/A00207.html> (accessed 2 July 2010), Compendium of Chemical Terminology Internet edition; International Union of Pure and Applied Chemistry (IUPAC). ketazines, <http://goldbook.iupac.org/K03377.html> (accessed 2 July 2010), Compendium of Chemical Terminology Internet edition; International Union of Pure and Applied Chemistry (IUPAC).
- ↑ 5.0 5.1 Day, A. C.; Whiting, M. C. Acetone hydrazone. Org. Synth. 1970, 50, 3, <http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0010>; Coll. Vol., 6, 10.
- ↑ Gilbert, E. C. Studies on Hydrazine. The Hydrolysis of Dimethylketazine and the Equilibrium between Hydrazine and Acetone. J. Am. Chem. Soc. 1929, 51 (11), 3394–3409. DOI: 10.1021/ja01386a032.
- ↑ 7.0 7.1 Schirmann, Jean-Pierre; Combroux, Jean; Delavarenne, Serge Yvon (Produits Chimiques Ugine Kuhlmann) Method for preparing azines and hydrazones. US Patent 3972878, issued 3 August 1976. Schirmann, Jean-Pierre; Tellier, Pierre; Mathais, Henri, et al. (Produits Chimiques Ugine Kuhlmann) Process for the preparation of hydrazine compounds. US Patent 3978049, issued 31 August 1976. Schirmann, Jean-Pierre; Combroux, Jean; Delavarenne, Serge Yvon (Produits Chimiques Ugine Kuhlmann) Method for making azines. US Patent 4093656, issued 6 June 1978.
- ↑ 8.0 8.1 Schirmann, Jean-Pierre; Combroux, Jean; Delavarenne, Serge Y. (Atochem) Preparation of a concentrated aqueous solution of hydrazine hydrate. US Patent 4724133, issued 9 February 1988.
- ↑ 9.0 9.1 Staudinger, H.; Gaule, Alice Vergleich der Stickstoff-Abspaltung bei verschiedenen aliphatischen Diazoverbindungen. Ber. Dtsch. Chem. Ges. 1916, 49 (2), 1897–1918. DOI: 10.1002/cber.19160490245.
- ↑ Day, A. C.; Raymond, P.; Southam, R. M.; Whiting, M. C. The preparation of secondary aliphatic diazo-compounds from hydrazones. J. Chem. Soc. C 1966, 467–69. DOI: 10.1039/J39660000467.
- ↑ Andrews, S. D.; Day, A. C.; Raymond, P.; Whiting, M. C. 2-Diazopropane. Org. Synth. 1970, 50, 27, <http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0392>; Coll. Vol., 6, 392.
- ↑ Eichenhofer, Kurt-Wilhelm; Schliebs, Reinhard (Bayer) Production of ketazines. US Patent 3965097, issued 22 June 1976.
- ↑ Holleman, A. F.; Wiberg, E. Inorganic Chemistry; Academic Press: San Diego, 2001; p 619. ISBN 0-12-352651-5.
- ↑ Nagata, Nobuhiro; Nishizawa, Chiharu; Kurai, Toshikiyo (Mitsubishi Gas Chemical Co.) Method of producing 1,2,4-triazole. US Patent 6002015, issued 14 December 1999.
- ↑ Gudkova, A. S.; Reutov, O. A.; Aleinikova, M. Ya. Izv. Akad. Nauk SSSR, Otdel. Khim. Nauk 1962 (8), 1382–87; Reactions of hydrazones and azines with metal salts 4. Reactions of azines of aldehydes and ketones with cupric salts. Russ. Chem. Bull. (Transl.) 1962, 11 (8), 1298–1302. DOI: 10.1007/BF00907973.
- ↑ Gudkova, A. S.; Aleinikova, M. Ya.; Reutov, O. A. Izv. Akad. Nauk SSSR, Ser. Khim. 1966 (5), 844–48; Reactions of hydrazones and azines with metal salts 5. Reactions of hydrazones and azines with mercuric halides. Russ. Chem. Bull. (Transl.) 1966, 15 (5), 807–11. DOI: 10.1007/BF00849376.
- ↑ King, Fiona; Nicholls, David Complex of titanium halides with acetone azine and its isomer 3,5,5-trimethyl-pyrazoline. Inorg. Chim. Acta 1978, 28, 55–58. DOI: 10.1016/S0020-1693(00)87413-7.
- ↑ Davis, William E., II; Li, Yongtao Analysis of hydrazine in drinking water by isotope dilution gas chromatography/tandem mass spectrometry with derivatization and liquid-liquid extraction. Anal. Chem. 2008, 80 (14), 5449–53. DOI: 10.1021/ac702536d.
- ↑ Sun, Mingjiang; Bai, Lin; Liu, David Q. A generic approach for the determination of trace hydrazine in drug substances using in situ derivatization-headspace GC–MS. J. Pharm. Biomed. Anal. 2009, 49 (2), 529–33. DOI: 10.1016/j.jpba.2008.11.009.
- ↑ 20.0 20.1 20.2 Rule R-5.6.6.3. In A Guide to IUPAC Nomenclature of Organic Compounds; IUPAC Recommendations 1993; Blackwell Science: Oxford, 1993; p 105. ISBN 0-632-03488-2, <http://www.acdlabs.com/iupac/nomenclature/93/r93_469.htm>.
- ↑ Draft Rule P-68.3.1.2.3. In Nomenclature of Organic Chemistry; IUPAC Provisional Recommendations 2004; IUPAC, 2004, <http://old.iupac.org/reports/provisional/abstract04/favre_310305.html>.
- ↑ Draft Rule P-53.6.2. In Nomenclature of Organic Chemistry; IUPAC Provisional Recommendations 2004; IUPAC, 2004, <http://old.iupac.org/reports/provisional/abstract04/favre_310305.html>.
- ↑ Rule C-923.2. In Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H; IUPAC Recommendations 1979; Pergamon: Oxford, 1979. ISBN 0-08022-369-9, <http://www.acdlabs.com/iupac/nomenclature/79/r79_623.htm>.
| Error creating thumbnail: Unable to save thumbnail to destination |
This page is currently licensed under the Creative Commons Attribution 3.0 Unported license and any later versions of that license. |