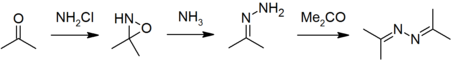Difference between revisions of "Bayer hydrazine process"
Physchim62 (talk | contribs) |
Physchim62 (talk | contribs) (→Process chemistry) |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | The '''Bayer hydrazine process''' is a modification of the [[Raschig process]] for the industrial production of [[hydrazine]]. Both processes are based around the [[oxidation]] of [[ammonia]] to hydrazine with [[hypochlorite]], but the Bayer process traps the hydrazine as [[acetone azine]] before [[Hydrolysis|hydrolyzing]] the [[azine]] in a separate step.<ref name="Bayer">{{citation | inventor1-last = Eichenhofer | inventor1-first = Kurt-Wilhelm | inventor2-last = Schliebs | inventor2-first = Reinhard | assignee = Bayer | title = Production of ketazines | country-code = US | patent-number = 3965097 | issue-date = 1976-06-22}}.</ref><ref name="Maxwell">{{citation | title = Synthetic nitrogen products: a practical guide to the products and processes | first = Gary R. | last = Maxwell | pages = | + | The '''Bayer hydrazine process''', also called the '''Bayer ketazine process''', is a modification of the [[Raschig process]] for the industrial production of [[hydrazine]]. Both processes are based around the [[oxidation]] of [[ammonia]] to hydrazine with [[hypochlorite]], but the Bayer process traps the hydrazine as [[acetone azine]] before [[Hydrolysis|hydrolyzing]] the [[azine]] in a separate step.<ref name="Bayer">{{citation | inventor1-last = Eichenhofer | inventor1-first = Kurt-Wilhelm | inventor2-last = Schliebs | inventor2-first = Reinhard | assignee = Bayer | title = Production of ketazines | country-code = US | patent-number = 3965097 | issue-date = 1976-06-22}}.</ref><ref name="Maxwell">{{citation | title = Synthetic nitrogen products: a practical guide to the products and processes | first = Gary R. | last = Maxwell | pages = 338–44 | publisher = Springer | year = 2004 | isbn = 0306482258}}.</ref><ref name="H&W">{{Holleman&Wiberg|page=619}}.</ref> The process is operated by [[Bayer]] at its Leverkusen plant in western Germany.<ref>{{citation | title = Material Flowsheet CHEMPAK LEV | url = http://www.chempark.com/medien/allgemein/downloads/VERBLEV20090430mFLogoENG.pdf | publisher = Bayer | date = 2009-04-28 | accessdate = 2010-07-03}}.</ref> |
==Process chemistry== | ==Process chemistry== | ||
| − | The oxidation of ammonia by hypochlorite proceeds by a [[chloramine]] intermediate:<ref name="C&W">{{Cotton&Wilkinson5th|page=317}}.</ref> | + | The [[oxidation]] of [[ammonia]] by [[hypochlorite]] proceeds by a [[chloramine]] intermediate:<ref name="C&W">{{Cotton&Wilkinson5th|page=317}}.</ref> |
:NH<sub>3</sub> + ClO<sup>−</sup> → NH<sub>2</sub>Cl + OH<sup>−</sup> | :NH<sub>3</sub> + ClO<sup>−</sup> → NH<sub>2</sub>Cl + OH<sup>−</sup> | ||
:NH<sub>3</sub> + NH<sub>2</sub>Cl + OH<sup>−</sup> → N<sub>2</sub>H<sub>4</sub> + Cl<sup>−</sup> + H<sub>2</sub>O | :NH<sub>3</sub> + NH<sub>2</sub>Cl + OH<sup>−</sup> → N<sub>2</sub>H<sub>4</sub> + Cl<sup>−</sup> + H<sub>2</sub>O | ||
| − | However, the hydrazine is prone to a [[disproportionation]] reaction in the presence of chloramine: | + | However, the [[hydrazine]] is prone to a [[disproportionation]] reaction in the presence of chloramine: |
| − | : | + | :2 NH<sub>2</sub>Cl + N<sub>2</sub>H<sub>4</sub> → 2 NH<sub>4</sub>Cl + N<sub>2</sub> |
| − | The disproportionation reaction is "rather fast once some hydrazine has been formed", and a [[gelatin]] inhibitor must be used in the Raschig process to obtain appreciable yields of hydrazine.<ref name="C&W"/> | + | The disproportionation reaction is "rather fast once some hydrazine has been formed", and a [[gelatin]] inhibitor must be used in the [[Raschig process]] to obtain appreciable yields of hydrazine.<ref name="C&W"/> |
| − | The Bayer process adds acetone to the reaction mixture to trap the hydrazine as acetone azine, which is inert to chloramine. The mechanism appears to be slightly different from the Raschig process, with chloramine reacting with acetone to for [[3,3-dimethyloxazidirine]], which then reacts with ammonia to form [[acetone hydrazone]]. The hydrazone then reacts with a further equivalent of acetone to form the azine, which is the isolated product.<ref name="Bayer"/> Typical process conditions are 35 | + | The Bayer process adds [[acetone]] to the reaction mixture to trap the hydrazine as [[acetone azine]], which is inert to chloramine. The mechanism appears to be slightly different from the Raschig process, with chloramine reacting with acetone to for [[3,3-dimethyloxazidirine]], which then reacts with ammonia to form [[acetone hydrazone]]. The hydrazone then reacts with a further equivalent of acetone to form the [[azine]], which is the isolated product.<ref name="Bayer"/> Typical process conditions are 35 °C and 2 bar, with a feed mix of NaOCl:Me<sub>2</sub>CO:NH<sub>3</sub> in a molar ratio of about 1:2:20.<ref name="Maxwell"/> |
[[File:Bayer hydrazine process.png|451px]] | [[File:Bayer hydrazine process.png|451px]] | ||
| − | The acetone azine is hydrolyzed in a separate step, by [[distillation]]. The hydrolysis is endothermic, | + | The acetone azine is [[Hydrolysis|hydrolyzed]] in a separate step, by [[distillation]]. The hydrolysis is [[endothermic]],<ref>{{citation | first = E. C. | last = Gilbert | title = Studies on Hydrazine. The Hydrolysis of Dimethylketazine and the Equilibrium between Hydrazine and Acetone | journal = J. Am. Chem. Soc. | year = 1929 | volume = 51 | issue = 11 | pages = 3394–3409 | doi = 10.1021/ja01386a032}}.</ref> and so requires an increase in temperature (and pressure) to shift the [[equilibrium]] in favour of the desired products: typical conditions are a pressure of 8 bar and temperatures of 130 °C at the base of the column and 179 °C at the top of the column. The hydrazine hydrate (30–45% [[aqueous solution]]) is run off from the base of the column, while the acetone is distilled off from the top of the column and recycled.<ref name="Atochem">{{citation | inventor1-first = Jean-Pierre | inventor1-last = Schirmann | inventor2-first = Jean | inventor2-last = Combroux | inventor3-first = Serge Y. | inventor3-last = Delavarenne | assignee = Atochem | country-code = US | patent-number = 4724133 | title = Preparation of a concentrated aqueous solution of hydrazine hydrate | issue-date = 1988-02-09}}.</ref> |
==Disadvantages== | ==Disadvantages== | ||
| − | The main disadvantage of the Bayer process, as with the Raschig process, is the production of two [[equivalent (chemistry)|equivalent]]s of [[sodium chloride]] for each equivalent of hydrazine. It takes about 3.5 kg of [[sodium hypochlorite]] to produce 1 kg of hydrazine.<ref name="Maxwell"/> | + | The main disadvantage of the Bayer process, as with the [[Raschig process]], is the production of two [[equivalent (chemistry)|equivalent]]s of [[sodium chloride]], which must be disposed of or recycled, for each equivalent of [[hydrazine]]. It takes about 3.5 kg of [[sodium hypochlorite]] to produce 1 kg of hydrazine, similar to the Raschig process as the two processes have the same overall [[stoichiometry]].<ref name="Maxwell"/> |
| + | |||
| + | The hydrolysis of the [[acetone azine]] is endothermic, but this energy cost is offset by the milder operating conditions of the Bayer process (compared with the Raschig process), and the much higher concentrations of [[hydrazine hydrate]] which are produced as the primary product. [[Azine]]s can also be used directly in [[organic synthesis]] as ''in situ'' hydrazine sources, as in a process for the production of the [[herbicide]] percursor [[1,2,4-triazole]].<ref>{{citation | inventor1-last = Nagata | inventor1-first = Nobuhiro | inventor2-last = Nishizawa | inventor2-first = Chiharu | inventor3-last = Kurai | inventor3-first = Toshikiyo | assignee = Mitsubishi Gas Chemical Co. | title = Method of producing 1,2,4-triazole | country-code = US | patent-number = 6002015 | issue-date = 1999-12-14}}.</ref> | ||
| + | |||
| + | Nevertheless, new hydrazine production facilities use the [[Pechiney-Udine-Kuhlmann process]], which is deemed to be more environmentally friendly as it avoids the use of hypochlorite as the [[oxidizing agent]] (and hence the production of sodium chloride waste).<ref name="Maxwell"/> | ||
==References== | ==References== | ||
Latest revision as of 18:50, 3 July 2010
The Bayer hydrazine process, also called the Bayer ketazine process, is a modification of the Raschig process for the industrial production of hydrazine. Both processes are based around the oxidation of ammonia to hydrazine with hypochlorite, but the Bayer process traps the hydrazine as acetone azine before hydrolyzing the azine in a separate step.[1][2][3] The process is operated by Bayer at its Leverkusen plant in western Germany.[4]
Process chemistry
The oxidation of ammonia by hypochlorite proceeds by a chloramine intermediate:[5]
- NH3 + ClO− → NH2Cl + OH−
- NH3 + NH2Cl + OH− → N2H4 + Cl− + H2O
However, the hydrazine is prone to a disproportionation reaction in the presence of chloramine:
- 2 NH2Cl + N2H4 → 2 NH4Cl + N2
The disproportionation reaction is "rather fast once some hydrazine has been formed", and a gelatin inhibitor must be used in the Raschig process to obtain appreciable yields of hydrazine.[5]
The Bayer process adds acetone to the reaction mixture to trap the hydrazine as acetone azine, which is inert to chloramine. The mechanism appears to be slightly different from the Raschig process, with chloramine reacting with acetone to for 3,3-dimethyloxazidirine, which then reacts with ammonia to form acetone hydrazone. The hydrazone then reacts with a further equivalent of acetone to form the azine, which is the isolated product.[1] Typical process conditions are 35 °C and 2 bar, with a feed mix of NaOCl:Me2CO:NH3 in a molar ratio of about 1:2:20.[2]
The acetone azine is hydrolyzed in a separate step, by distillation. The hydrolysis is endothermic,[6] and so requires an increase in temperature (and pressure) to shift the equilibrium in favour of the desired products: typical conditions are a pressure of 8 bar and temperatures of 130 °C at the base of the column and 179 °C at the top of the column. The hydrazine hydrate (30–45% aqueous solution) is run off from the base of the column, while the acetone is distilled off from the top of the column and recycled.[7]
Disadvantages
The main disadvantage of the Bayer process, as with the Raschig process, is the production of two equivalents of sodium chloride, which must be disposed of or recycled, for each equivalent of hydrazine. It takes about 3.5 kg of sodium hypochlorite to produce 1 kg of hydrazine, similar to the Raschig process as the two processes have the same overall stoichiometry.[2]
The hydrolysis of the acetone azine is endothermic, but this energy cost is offset by the milder operating conditions of the Bayer process (compared with the Raschig process), and the much higher concentrations of hydrazine hydrate which are produced as the primary product. Azines can also be used directly in organic synthesis as in situ hydrazine sources, as in a process for the production of the herbicide percursor 1,2,4-triazole.[8]
Nevertheless, new hydrazine production facilities use the Pechiney-Udine-Kuhlmann process, which is deemed to be more environmentally friendly as it avoids the use of hypochlorite as the oxidizing agent (and hence the production of sodium chloride waste).[2]
References
- ↑ 1.0 1.1 Eichenhofer, Kurt-Wilhelm; Schliebs, Reinhard (Bayer) Production of ketazines. US Patent 3965097, issued 22 June 1976.
- ↑ 2.0 2.1 2.2 2.3 Maxwell, Gary R. Synthetic nitrogen products: a practical guide to the products and processes; Springer, 2004; pp 338–44. ISBN 0306482258.
- ↑ Holleman, A. F.; Wiberg, E. Inorganic Chemistry; Academic Press: San Diego, 2001; p 619. ISBN 0-12-352651-5.
- ↑ Material Flowsheet CHEMPAK LEV; Bayer, 2009-04-28, <http://www.chempark.com/medien/allgemein/downloads/VERBLEV20090430mFLogoENG.pdf>. (accessed 3 July 2010).
- ↑ 5.0 5.1 Cotton, F. Albert; Wilkinson, Geoffrey Advanced Inorganic Chemistry, 5th ed.; Wiley-Interscience: New York, 1988; p 317. ISBN 0-471-84997-9.
- ↑ Gilbert, E. C. Studies on Hydrazine. The Hydrolysis of Dimethylketazine and the Equilibrium between Hydrazine and Acetone. J. Am. Chem. Soc. 1929, 51 (11), 3394–3409. DOI: 10.1021/ja01386a032.
- ↑ Schirmann, Jean-Pierre; Combroux, Jean; Delavarenne, Serge Y. (Atochem) Preparation of a concentrated aqueous solution of hydrazine hydrate. US Patent 4724133, issued 9 February 1988.
- ↑ Nagata, Nobuhiro; Nishizawa, Chiharu; Kurai, Toshikiyo (Mitsubishi Gas Chemical Co.) Method of producing 1,2,4-triazole. US Patent 6002015, issued 14 December 1999.
| Error creating thumbnail: Unable to save thumbnail to destination |
This page is currently licensed under the Creative Commons Attribution 3.0 Unported license and any later versions of that license. |