Difference between revisions of "Substance:Benzil"
(Expand) |
(Add MS links) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:Benzil.png|200px|Structure of benzil | + | {| class="wikitable" border="0" style="float: right;" |
| + | |- | ||
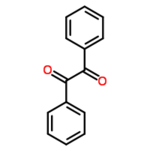
| + | |<!--column1-->[[File:Benzil.png|150px|Structure of benzil]] | ||
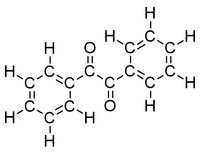
| + | |<!--column2-->[[File:BenzilFullStructure.png|200px|Structure of benzil with all atoms shown]] | ||
| + | |}<!--end wikitable--> | ||
| + | |||
*'''[http://www.chemspider.com/RecordView.aspx?rid=01fff70a-185b-4743-9c31-e2eec8d9b886 Main ChemSpider page]''' | *'''[http://www.chemspider.com/RecordView.aspx?rid=01fff70a-185b-4743-9c31-e2eec8d9b886 Main ChemSpider page]''' | ||
*Molecular formula: C<sub>14</sub>H<sub>10</sub>O<sub>2</sub> | *Molecular formula: C<sub>14</sub>H<sub>10</sub>O<sub>2</sub> | ||
| Line 9: | Line 14: | ||
*Solubility properties: Not available | *Solubility properties: Not available | ||
*Safety sheet: [http://msds.chem.ox.ac.uk/BE/benzil.html From Oxford University] | *Safety sheet: [http://msds.chem.ox.ac.uk/BE/benzil.html From Oxford University] | ||
| − | *Spectra: [http://webbook.nist.gov/cgi/cbook.cgi?ID=C134816&Units=SI&Type=IR-SPEC&Index=1#IR-SPEC IR], <sup>1</sup>H NMR, [http://www.ebi.ac.uk/nmrshiftdb/portal/js_pane/P-Results/nmrshiftdbaction/showDetailsFromHome/molNumber/10005895 <sup>13</sup>C NMR], MS. Also check on [http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng SDBS]. | + | *Spectra: [http://webbook.nist.gov/cgi/cbook.cgi?ID=C134816&Units=SI&Type=IR-SPEC&Index=1#IR-SPEC IR], <sup>1</sup>H NMR, [http://www.ebi.ac.uk/nmrshiftdb/portal/js_pane/P-Results/nmrshiftdbaction/showDetailsFromHome/molNumber/10005895 <sup>13</sup>C NMR], MS ([http://www.massbank.jp/jsp/FwdRecord.jsp?type=disp&id=JP001411 1], [http://www.massbank.jp/jsp/FwdRecord.jsp?type=disp&id=JP009582 2], [http://www.massbank.jp/jsp/FwdRecord.jsp?type=disp&id=JP010530 3], [http://www.massbank.jp/jsp/FwdRecord.jsp?type=disp&id=JP009582 4]). Also check on [http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng SDBS]. |
==From Wikipedia== | ==From Wikipedia== | ||
| − | '''Benzil''' (systematically known as 1,2-diphenyl-1,2-ethanedione) is the | + | '''Benzil''' (systematically known as 1,2-diphenyl-1,2-ethanedione) is the organic compound with the formula (C<sub>6</sub>H<sub>5</sub>CO)<sub>2</sub>, generally abbreviated (PhCO)<sub>2</sub>. This yellow solid is one of the most common diketones. Its main use is as a photoinitiator in polymer chemistry.<ref name=Ullmann>Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Wienheim. {{DOI|10.1002/14356007.a15_077}}</ref> [http://en.wikipedia.org/wiki/Benzil Read more...] or [http://en.wikipedia.org/w/index.php?title=Benzil&action=edit edit at Wikipedia]. |
==References== | ==References== | ||
Latest revision as of 01:03, 7 September 2010
|
|
- Main ChemSpider page
- Molecular formula: C14H10O2
- Molar mass: 210.228
- CAS Registry Number: 134-81-6
- IUPAC name: 1,2-diphenylethane-1,2-dione
- Appearance: Yellow crystals or powder
- Melting point: 94-95 °C
- Solubility properties: Not available
- Safety sheet: From Oxford University
- Spectra: IR, 1H NMR, 13C NMR, MS (1, 2, 3, 4). Also check on SDBS.
From Wikipedia
Benzil (systematically known as 1,2-diphenyl-1,2-ethanedione) is the organic compound with the formula (C6H5CO)2, generally abbreviated (PhCO)2. This yellow solid is one of the most common diketones. Its main use is as a photoinitiator in polymer chemistry.[1] Read more... or edit at Wikipedia.
References
- ↑ Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Wienheim. DOI:10.1002/14356007.a15_077