Difference between revisions of "Substance:Ethyl acetate"
(Start page) |
m (Fixed) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{| class="wikitable" border="0" style="float: right;" | {| class="wikitable" border="0" style="float: right;" | ||
|- | |- | ||
| − | |<!--column1-->[[File: | + | |<!--column1-->[[File:EthylAcetate.png|100px|Structure of ethyl acetate]] |
|<!--column2-->[[File:EthylAcetateFullStructure.png|200px|Structure of ethyl acetate with all atoms shown]] | |<!--column2-->[[File:EthylAcetateFullStructure.png|200px|Structure of ethyl acetate with all atoms shown]] | ||
|}<!--end wikitable--> | |}<!--end wikitable--> | ||
| Line 9: | Line 9: | ||
*Molar mass: 88.1051 | *Molar mass: 88.1051 | ||
*CAS Registry Number: 141-78-6 | *CAS Registry Number: 141-78-6 | ||
| − | *IUPAC name: | + | *IUPAC name: Ethyl acetate |
| − | + | *Appearance: Colorless liquid with an ether-like, fruity odor. | |
| − | + | *Boiling point: 77 °C | |
| − | *Appearance: | ||
| − | * | ||
*Solubility properties: Not available | *Solubility properties: Not available | ||
| − | *Safety sheet: [http:// | + | *Safety sheet: [http://www.chemadvisor.com/SymyxMSDS/ohsdoc.pl?OHSNUMBER=OHS08750&DOCTYPE=SUMMARY From ChemAdvisor] |
| − | *Spectra: [http://webbook.nist.gov/cgi/cbook.cgi?ID= | + | *Spectra: [http://webbook.nist.gov/cgi/cbook.cgi?ID=C141786&Units=SI&Type=IR-SPEC&Index=1#IR-SPEC IR], [http://www.ebi.ac.uk/nmrshiftdb/portal/js_pane/P-Results/nmrshiftdbaction/showDetailsFromHome/molNumber/10008694 <sup>1</sup>H NMR], [http://www.ebi.ac.uk/nmrshiftdb/portal/js_pane/P-Results/nmrshiftdbaction/showDetailsFromHome/molNumber/10008694 <sup>13</sup>C NMR], [http://www.massbank.jp/jsp/FwdRecord.jsp?type=disp&id=JP001797 MS]. Also check on [http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng SDBS]. |
==From Wikipedia== | ==From Wikipedia== | ||
| − | + | Ethyl acetate (systematically, ethyl ethanoate, commonly abbreviated EtOAc or EA) is the organic compound with the formula CH<sub>3</sub>COOCH<sub>2</sub>CH<sub>3</sub>. This colorless liquid has a characteristic sweet smell (similar to pear drops) like certain glues or nail polish removers, in which it is used. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent. In 1985, about 400,000 tons were produced yearly in Japan, North America, and Europe combined. In 2004, an estimated 1.3M tons were produced worldwide. [http://en.wikipedia.org/wiki/Ethyl_acetate Read more...] or [http://en.wikipedia.org/w/index.php?title=Ethyl_acetate&action=edit edit at Wikipedia]. | |
==References== | ==References== | ||
Latest revision as of 00:21, 10 September 2010
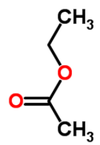
|
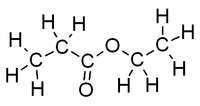
|
- Main ChemSpider page
- Molecular formula: C4H8O2
- Molar mass: 88.1051
- CAS Registry Number: 141-78-6
- IUPAC name: Ethyl acetate
- Appearance: Colorless liquid with an ether-like, fruity odor.
- Boiling point: 77 °C
- Solubility properties: Not available
- Safety sheet: From ChemAdvisor
- Spectra: IR, 1H NMR, 13C NMR, MS. Also check on SDBS.
From Wikipedia
Ethyl acetate (systematically, ethyl ethanoate, commonly abbreviated EtOAc or EA) is the organic compound with the formula CH3COOCH2CH3. This colorless liquid has a characteristic sweet smell (similar to pear drops) like certain glues or nail polish removers, in which it is used. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent. In 1985, about 400,000 tons were produced yearly in Japan, North America, and Europe combined. In 2004, an estimated 1.3M tons were produced worldwide. Read more... or edit at Wikipedia.