Difference between revisions of "Nakada's synthesis of digitoxigenin"
(Add cats) |
Physchim62 (talk | contribs) |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
==Cardiac drug structure== | ==Cardiac drug structure== | ||
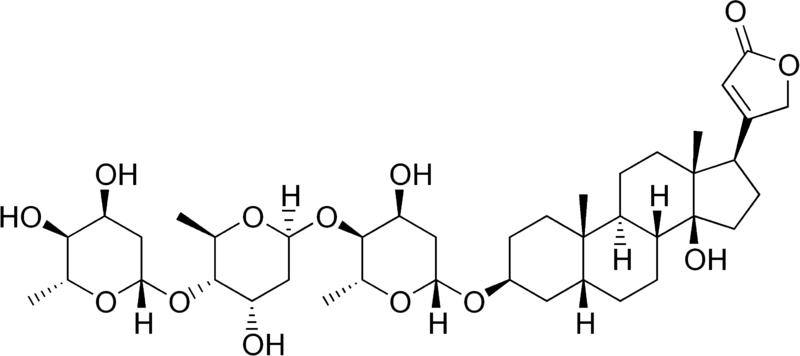
| − | A glycoside consists of part sugar and part nonsugar. The sugar portion is called the glycone and the nonsugar portion is called the aglycone, or genin. Digitoxigenin is the aglycone of | + | A glycoside consists of part sugar and part nonsugar. The sugar portion is called the glycone and the nonsugar portion is called the aglycone, or genin. Digitoxigenin is the aglycone of [[digitoxin]]. |
| − | + | [[File:Digitoxin.png|800px|center]] | |
| − | [[ | ||
| − | |||
| − | |||
| − | |||
| − | [[ | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Retrosynthetic Analysis== | ==Retrosynthetic Analysis== | ||
| Line 31: | Line 14: | ||
==Synthesis of Part A== | ==Synthesis of Part A== | ||
| − | [[Image:digitoxigeninsynthesisparta.png| | + | [[Image:digitoxigeninsynthesisparta.png|800px|center]] |
| − | #Ethylene ketal formation (protecting group), addition of protecting group | + | #Ethylene ketal formation (protecting group), addition of TBS protecting group |
| − | #Conversion of an alkene to an alcohol and protection of the alcohol with | + | #Conversion of an alkene to an alcohol and protection of the alcohol with TBDPSCl, ketal hydrolyzed to ketone with base |
| − | # | + | #[[Wolff–Kishner reduction]], Conversion to -I, [[Suzuki coupling reaction]], removal of protecting groups TBDPSO and TBSO |
#Protection of the primary alcohol with a TBS protecting group, Oxidation of the secondary alcohol to a ketone | #Protection of the primary alcohol with a TBS protecting group, Oxidation of the secondary alcohol to a ketone | ||
#Removal of the TBS protecting group, Conversion of the alcohol to a bromine | #Removal of the TBS protecting group, Conversion of the alcohol to a bromine | ||
| − | #Formation of a ketal from the ketone and final step of product A | + | #Formation of a ketal from the ketone and final step of product A. |
==Synthesis of Part B== | ==Synthesis of Part B== | ||
| − | [[Image:digitoxigeninsynthesispartb.png| | + | [[Image:digitoxigeninsynthesispartb.png|800px|center]] |
| − | #The first step | + | #The first step is a catalytic asymmetric intramolecular cyclopropanation |
| − | #The cyclopropane ring | + | #The cyclopropane ring is opened and the phenylthio group/phenylsulfonyl group removed. |
| − | #The starting material | + | #The starting material is first reduced with L-selectride to form the correct stereoisomer. The hydroxyl is then protected with a TBDPS protecting group. |
| − | #Allylic oxidation | + | #Allylic oxidation is performed using a reagent reported by Ochiai to form retrosynthetic piece B. |
==Convergent Steps== | ==Convergent Steps== | ||
| − | [[Image:digitoxigeninfinalsynthesis.png| | + | [[Image:digitoxigeninfinalsynthesis.png|800px|center]] |
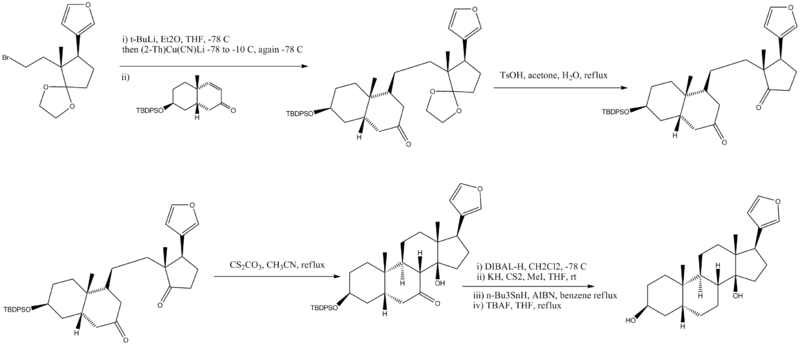
#Cuprate with lithium produces a single diastereomer | #Cuprate with lithium produces a single diastereomer | ||
| Line 62: | Line 45: | ||
*Desai, Umesh. [http://www.people.vcu.edu/~urdesai/car.htm www.people.vcu.edu/~urdesai/car.htm], accessed 16 April 2008. | *Desai, Umesh. [http://www.people.vcu.edu/~urdesai/car.htm www.people.vcu.edu/~urdesai/car.htm], accessed 16 April 2008. | ||
*Haux, J. ''Medical Hypotheses'' (1999) 53(6), 543-54. [http://dx.doi.org/10.1054/mehy.1999.0985 doi:10.1054/mehy.1999.0985] | *Haux, J. ''Medical Hypotheses'' (1999) 53(6), 543-54. [http://dx.doi.org/10.1054/mehy.1999.0985 doi:10.1054/mehy.1999.0985] | ||
| + | |||
| + | [[Category:Total syntheses]] | ||
| + | [[Category:Chemistry 444 pages]] | ||
| + | |||
| + | {{CC-BY-SA-3.0 and GFDL-1.2}} | ||
Latest revision as of 14:38, 16 August 2009
Digitoxin and digoxin make up the drug digitalis, which is used as a cardiac drug to treat cardiac congestion and cardiac arrythmias. The mechanism within the heart involves increasing intracellular Na+ and Ca2+ while decreasing intracellular K+. The increased Ca2+ promotes muscle contraction and cardiac contractile force. Digitalis has also been reported to exhibit anti-cancer activity.
Contents
Cardiac drug structure
A glycoside consists of part sugar and part nonsugar. The sugar portion is called the glycone and the nonsugar portion is called the aglycone, or genin. Digitoxigenin is the aglycone of digitoxin.
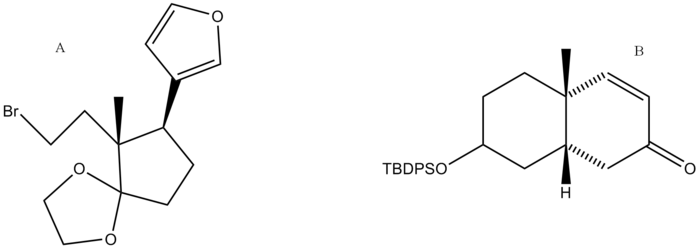
Retrosynthetic Analysis
The synthesis of digitoxigenin was developed by Honma and Nakada. A retrosynthetic analysis was performed and the final product was broken down into two major retrosynthetic pieces, shown below (pieces A and B).
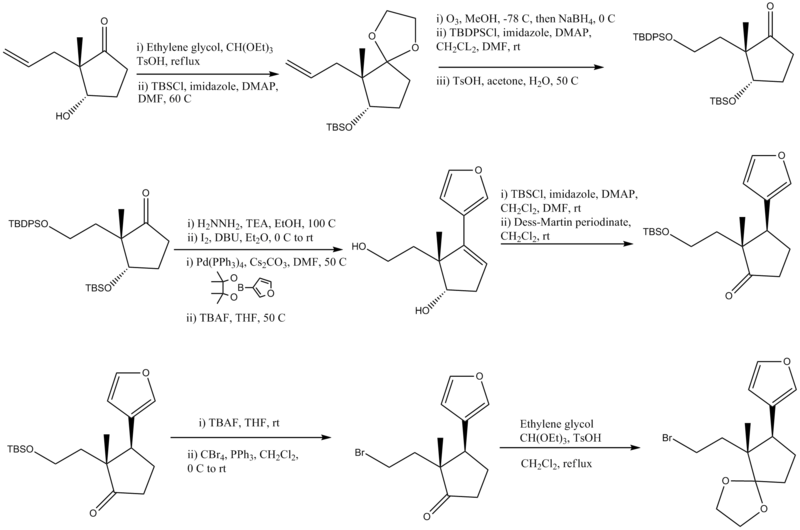
Synthesis of Part A
- Ethylene ketal formation (protecting group), addition of TBS protecting group
- Conversion of an alkene to an alcohol and protection of the alcohol with TBDPSCl, ketal hydrolyzed to ketone with base
- Wolff–Kishner reduction, Conversion to -I, Suzuki coupling reaction, removal of protecting groups TBDPSO and TBSO
- Protection of the primary alcohol with a TBS protecting group, Oxidation of the secondary alcohol to a ketone
- Removal of the TBS protecting group, Conversion of the alcohol to a bromine
- Formation of a ketal from the ketone and final step of product A.
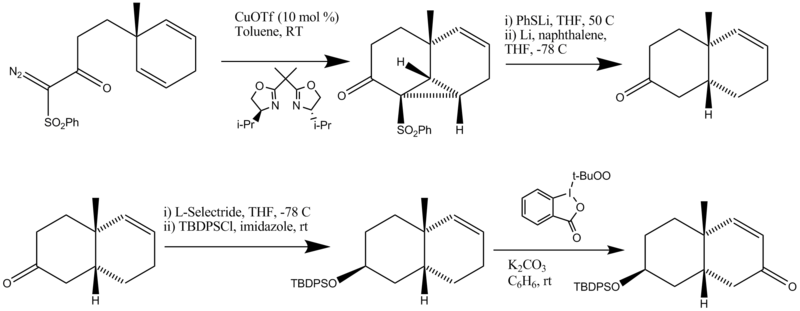
Synthesis of Part B
- The first step is a catalytic asymmetric intramolecular cyclopropanation
- The cyclopropane ring is opened and the phenylthio group/phenylsulfonyl group removed.
- The starting material is first reduced with L-selectride to form the correct stereoisomer. The hydroxyl is then protected with a TBDPS protecting group.
- Allylic oxidation is performed using a reagent reported by Ochiai to form retrosynthetic piece B.
Convergent Steps
- Cuprate with lithium produces a single diastereomer
- Ethylene ketal removed under acidic conditions
- Intramolecular aldol reaction
- Deoxygenation in four steps, TBDPS protecting group removed
References
- Honma, Masahiro and Nakada, Masahisa. Tet. Letters 48(2007) 1541-1544. doi:10.1016/j.tetlet.2007.01.024
- Desai, Umesh. www.people.vcu.edu/~urdesai/car.htm, accessed 16 April 2008.
- Haux, J. Medical Hypotheses (1999) 53(6), 543-54. doi:10.1054/mehy.1999.0985
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page is currently licensed under both the Creative Commons Attribution–Share Alike 3.0 Unported license and the GNU Free Distribution License version 1.2 and any later versions of that license. It may be used in GFDL 1.3 documents. |