Difference between revisions of "Sarsasapogenin"
Physchim62 (talk | contribs) |
Physchim62 (talk | contribs) |
||
| (15 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{chembox | {{chembox | ||
| ImageFile = Sarsasapogenin.png | | ImageFile = Sarsasapogenin.png | ||
| − | | IUPACName = ( | + | | IUPACName = (5β,25''S'')-spirostan-3β-ol |
| Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| InChI={{InChIwrap|1/C27H44O3/c1-16-7-12-27 (29-15-16)17(2)24-23(30-27)14- 22-20-6-5-18-13-19(28)8-10-25( 18,3)21(20)9-11-26(22,24)4/h16 -24,28H,5-15H2,1-4H3/t16-,17-, 18+,19-,20+,21-,22-,23-,24-,25 -,26-,27+/m0/s1}} | | InChI={{InChIwrap|1/C27H44O3/c1-16-7-12-27 (29-15-16)17(2)24-23(30-27)14- 22-20-6-5-18-13-19(28)8-10-25( 18,3)21(20)9-11-26(22,24)4/h16 -24,28H,5-15H2,1-4H3/t16-,17-, 18+,19-,20+,21-,22-,23-,24-,25 -,26-,27+/m0/s1}} | ||
| Line 16: | Line 16: | ||
| MolarMass = 416.64 g/mol | | MolarMass = 416.64 g/mol | ||
| MeltingPt = 199–199.5 °C | | MeltingPt = 199–199.5 °C | ||
| + | | Solubility1 = soluble | ||
| + | | Solvent1 = ethanol | ||
}} | }} | ||
}} | }} | ||
| Line 23: | Line 25: | ||
Sarsasapogenin is unusual in that it has a ''cis''-linkage between rings A and B of the steroid nucleus, as opposed to the more usual ''trans''-linkage found in other saturated steroids. This 5β configuration is biologically significant, as a specific enzyme – [[sarsasapogenin 3β-glucosyltransferase]] – is found in several plants for the glycosylation of sarsasapogenin.<ref>{{citation | last1 = Paczkowski | first1 = Cezary | last2 = Wojciechowski | first2 = Zdzisław A. | title = The occurrence of UDPG-dependent glucosyltransferase specific for sarsasapogenin in ''Asparagus officinalis'' | journal = Phytochemistry | volume = 27 | issue = 9 | year = 1988 | pages = 2743–47 | doi = 10.1016/0031-9422(88)80654-X}}.</ref> The (''S'')-configuration at C-25 is also in contrast to other spirostan sapogenins: the epimer with a (25''R'')-configuration is known as [[smilagenin]]. | Sarsasapogenin is unusual in that it has a ''cis''-linkage between rings A and B of the steroid nucleus, as opposed to the more usual ''trans''-linkage found in other saturated steroids. This 5β configuration is biologically significant, as a specific enzyme – [[sarsasapogenin 3β-glucosyltransferase]] – is found in several plants for the glycosylation of sarsasapogenin.<ref>{{citation | last1 = Paczkowski | first1 = Cezary | last2 = Wojciechowski | first2 = Zdzisław A. | title = The occurrence of UDPG-dependent glucosyltransferase specific for sarsasapogenin in ''Asparagus officinalis'' | journal = Phytochemistry | volume = 27 | issue = 9 | year = 1988 | pages = 2743–47 | doi = 10.1016/0031-9422(88)80654-X}}.</ref> The (''S'')-configuration at C-25 is also in contrast to other spirostan sapogenins: the epimer with a (25''R'')-configuration is known as [[smilagenin]]. | ||
| − | Sarsasapogenin has been used as a starting material for the synthesis of other steroids.<ref>{{citation | inventor1-last = Dryden, Jr. | inventor1-first = Hugh L. | inventor2-last = Markos | inventor2-first = Charles S. | assignee = Searle & Co. | country-code = US | patent-number = 4057543 | publication-date = 1977-11-08}}.</ref> It has also attracted pharmaceutical interest in its own right,<ref>{{citation | inventor1-last = Applezweig | inventor1-first = Norman | assignee = Progenics Inc. | country-code = US | patent-number = 4680289 | publication-date = 1987-07-14}}.</ref> and is found in the rhizome of ''Anemarrhena asphodeloides'', used in Chinese tradition medicine (知母, ''zhī mǔ''), from which it is extracted commercially.<ref name="Wilshire">{{citation | url = http://www.wilshiretechnologies.com/steroidal_saponins_and_sapogenins_pdf/sarsasapogenin.pdf | title = Sarsasapogenin | publisher = Wilshire Technologies | accessdate = 2010-03-07}}.</ref> | + | Sarsasapogenin has been used as a starting material for the synthesis of other steroids.<ref>{{citation | inventor1-last = Dryden, Jr. | inventor1-first = Hugh L. | inventor2-last = Markos | inventor2-first = Charles S. | assignee = Searle & Co. | title = Process for the preparation of 17β-hydroxy-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone | country-code = US | patent-number = 4057543 | publication-date = 1977-11-08}}.</ref> It has also attracted pharmaceutical interest in its own right,<ref name="US4680289">{{citation | inventor1-last = Applezweig | inventor1-first = Norman | assignee = Progenics Inc. | title = Treatment of obesity and diabetes using sapogenins | country-code = US | patent-number = 4680289 | publication-date = 1987-07-14}}.</ref><ref name="US6812213">{{citation | inventor1-last = Xia | inventor1-first = Zongqin | inventor2-last = Hu | inventor2-first = Yaer | inventor3-last = Rubin | inventor3-first = Ian | inventor4-last = Brostoff | inventor4-first = Jonathan | inventor5-last = Whittle | inventor5-first = Brian | inventor6-last = Wang | inventor6-first = Weijun | inventor7-last = Gunning | inventor7-first = Phil | assignee = Phytopharm plc | title = Steroidal sapogenins and their derivatives for treating alzheimer's disease | country-code = US | patent-number = 6812213 | publication-date = 2002-12-19}}.</ref><ref name="Yaer">{{citation | first1 = Yaer | last1 = Hu | first2 = Zongqin | last2 = Xia | first3 = Qixiang | last3 = Sun | first4 = Antonia | last4 = Orsi | first5 = Daryl | last5 = Rees | title = A new approach to the pharmacological regulation of memory: Sarsasapogenin improves memory by elevating the low muscarinic acetylcholine receptor density in brains of memory-deficit rat models | journal = Brain Research | year = 2005 | volume = 1060 | issue = 1–2 | pages = 26–39 | doi = 10.1016/j.brainres.2005.08.019}}.</ref> and is found in the rhizome of ''[[Anemarrhena asphodeloides]]'', used in Chinese tradition medicine (知母, ''zhī mǔ''), from which it is extracted commercially.<ref name="Wilshire">{{citation | url = http://www.wilshiretechnologies.com/steroidal_saponins_and_sapogenins_pdf/sarsasapogenin.pdf | title = Sarsasapogenin | publisher = Wilshire Technologies | accessdate = 2010-03-07}}.</ref> |
| + | |||
| + | ==Occurrence and isolation== | ||
| + | Sarsasapogenin is found as a [[glycoside]] – with one or more sugar units attached to the hydroxyl group, known as a [[saponin]] – in the roots of many species of [[monocotyledon]]ous plant, in particular:<ref name="US6812213"/> | ||
| + | |||
| + | [[Smilacaceae]] | ||
| + | *''[[Smilax]]'' sp. | ||
| + | **''[[Smilax regelii]]'' <small>Kilip & C. V. Morton</small> (Honduran sarsapilla) | ||
| + | ***''[[Smilax ornata]]'' <small>Hook.f.</small> (Jamaican sarsapilla, synonym of ''S. regelii'') | ||
| + | **''[[Smilax aristolochiaefolia]]'' <small>Mill.</small> (American sarsapilla) | ||
| + | **''[[Smilax aspera]]'' <small>L.</small> (Spanish sarsapilla) | ||
| + | **''[[Smilax glabra]]'' <small>Roxb.</small> (in Chinese, ''tǔfúlíng'' 土茯苓) | ||
| + | **''[[Smilax febrifuga]]'' <small>Kunth</small> (Ecuadorian or Peruvian sarsapilla) | ||
| + | [[Asparagaceae]] | ||
| + | *''[[Asparagus]]'' sp. | ||
| + | [[Agavaceae]] | ||
| + | *''[[Anemarrhena]]'' sp. | ||
| + | **''[[Anemarrhena asphodeloides]]'' <small>Bunge</small>(in Chinese, ''zhī mǔ'' 知母) | ||
| + | *''[[Yucca]]'' sp. | ||
| + | **''[[Yucca schidigera]]'' <small>Roezl ex Ortges</small> (Mojave yucca) | ||
| + | **''[[Yucca brevifolia]]'' <small>Enulm.</small> (Joshua tree) | ||
| + | *''[[Agave]]'' sp. | ||
| + | |||
| + | The sarsasapogenin saponin can be extracted from the dried powdered root with 95% [[ethanol]]. After removal of the fat from the resulting gum, the glycosidic linkage is [[Hydrolysis|hydrolyzed]] with [[hydrochloric acid]] (approx. 2 M) and the resulting crude steroid is [[Recrystallization|recrystallized]] from anhydrous [[acetone]]. The yield of pure sarsasapogenin from 225 kg of ''Smilax'' root is reported to be about 450 grams.<ref name="Jacobs"/> | ||
| + | |||
| + | ==History== | ||
| + | Sarsasapogenin was first isolated in 1914 from Sarsapilla root.<ref name="Power"/> Although it was known to have three oxygen atoms, of which only one is a hydroxyl group, the structure of the side chain remained unclear for many years. Tschesche and Hagedorn proposed an unreactive double [[tetrahydrofuran]] structure based on degradation studies which indicated an [[ether]] oxygen atom attached to C-16.<ref>{{citation | last1 = Tschesche | first1 = R. | last2 = Hagedorn | first2 = A. | title = Über neutrale Saponine, II. Mitteil.: Abbau eines Genins der neutralen Sapogenine zu einem Gallensäure-Derivat | journal = Ber. Dtsch. Chem. Ges. | year = 1935 | volume = 68 | page = 1412–20 | doi = 10.1002/cber.19350680736}}.</ref> The true nature of the side chain – a [[ketone]] [[spiro]] [[acetal]] – was discovered by [[Russell Earl Marker|Russell Marker]] in 1939, when he succeeded in opening the six-membered [[pyran]] ring with [[acetic anhydride]].<ref name="LIII"/> Marker found that almost the entire side chain could be cleaved in three steps, a process now known as the [[Marker degradation]]. | ||
| + | |||
| + | Marker was able to convert sarsasapogenin into [[pregane-3,20-diol]]<ref name="LXXXI">{{citation | title = Sterols. LXXXI. Conversion of Sarsasapogenin to Pregnanediol-3(α),20(α) | first1 = Russell E. | last1 = Marker | authorlink1 = Russell Earl Marker | first2 = Ewald | last2 = Rohrmann | journal = J. Am. Chem. Soc. | year = 1939 | volume = 61 | isuue = 12 pages = 3592–93 | doi = 10.1021/ja01267a513}}. {{citation | title = Sterols. LXXXVIII. Pregnanediols from Sarsasapogenin | first1 = Russell E. | last1 = Marker | authorlink1 = Russell Earl Marker | first2 = Ewald | last2 = Rohrmann | journal = J. Am. Chem. Soc. | year = 1940 | volume = 62 | issue = 3 | pages = 518–20 | doi = 10.1021/ja01860a017}}.</ref> (a [[progesterone]] analogue) and [[testosterone]].<ref name="CV">{{citation | title = Sterols. CV. The Preparation of Testosterone and Related Compounds from Sarsasapogenin and Diosgenin | first = Russell E. | last = Marker | authorlink = Russell Earl Marker | journal = J. Am. Chem. Soc. | year = 1940 | volume = 62 | issue = 9 | pages = 2543–47 | doi = 10.1021/ja01866a077}}.</ref> However, for large scale production of steroid hormones, it proved more convenient to use [[diosgenin]] (extracted from the Mexican yam ''[[Dioscorea mexicana]]'') as the starting material, as it contains a double bond in the steroid nucleus.<ref>{{citation | title = Sterols. C. Diosgenin | first1 = Russell E. | last1 = Marker | authorlink1 = Russell Earl Marker | first2 = Takeo | last2 = Tsukamoto | first3 = D. L. | last3 = Turner | journal = J. Am. Chem. Soc. | year = 1940 | volume = 62 | issue = 9 | pages = 2525–32 | doi = 10.1021/ja01866a072}}.</ref> | ||
| + | |||
| + | ==Pharmacological interest== | ||
| + | Sarsasapogenin and its C-25 epimer [[smilagenin]] lowered blood sugar and reversed diabetic weight gain in experiments with in mice with a mutant [[diabetes]] gene (''db'').<ref name="US4680289"/> Both steroids also halted the decline in [[muscarinic acetylcholine receptor]]s (mAChRs) in animal models of [[Alzheimer's disease]].<ref name="US6812213"/><ref name="Yaer"/> In both cases, the effects seem to be specific to the 5β-configuration, the ''cis''-linkage between rings A and B, as [[diosgenin]] (with a Δ<sup>5</sup> double bond which can be hydrogenated in the body) had much lower anti-diabetic activity<ref name="US4680289"/> (and no significant effect on mAChRs<ref name="US6812213"/>) while [[tigogenin]] (the 5α-epimer of smilagenin) showed no effect at all in either study.<ref name="US4680289"/><ref name="US6812213"/> | ||
==References== | ==References== | ||
| − | {{reflist}} | + | {{reflist|2}} |
[[Category:Spirostan steroids]] | [[Category:Spirostan steroids]] | ||
{{CC-BY-3.0}} | {{CC-BY-3.0}} | ||
Latest revision as of 21:30, 8 March 2010
| Sarsasapogenin | |
|---|---|
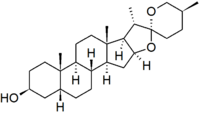
| |
| IUPAC name | (5β,25S)-spirostan-3β-ol |
| Identifiers | |
| InChI | InChI=1/C27H44O3/c1-16-7-12-27 (29-15-16)17(2)24-23(30-27)14- 22-20-6-5-18-13-19(28)8-10-25( 18,3)21(20)9-11-26(22,24)4/h16 -24,28H,5-15H2,1-4H3/t16-,17-, 18+,19-,20+,21-,22-,23-,24-,25 -,26-,27+/m0/s1 |
| InChIKey | GMBQZIIUCVWOCD-WWASVFFGBR |
| Standard InChI | InChI=1S/C27H44O3/c1-16-7-12-2 7(29-15-16)17(2)24-23(30-27)14 -22-20-6-5-18-13-19(28)8-10-25 (18,3)21(20)9-11-26(22,24)4/h1 6-24,28H,5-15H2,1-4H3/t16-,17- ,18+,19-,20+,21-,22-,23-,24-,2 5-,26-,27+/m0/s1 |
| Standard InChIKey | GMBQZIIUCVWOCD-WWASVFFGSA-N |
| CAS number | [] |
| EC number | |
| ChemSpider | |
| Properties[1] | |
| Chemical formula | C27H44O3 |
| Molar mass | 416.64 g/mol |
| Melting point |
199–199.5 °C |
| Solubility in ethanol | soluble |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Sarsasapogenin is a steroidal sapogenin, that is the aglycosidic portion of a plant saponin. It is named after sarsaparilla (Smilax sp.),[2] a family of climbing plants found in subtropical regions. It was one of the first sapogenins to be identified,[2] and the first spirostan steroid to be identified as such.[3] The identification of the spirostan structure, with its ketone spiro acetal functionality, was fundamental in the development of the Marker degradation, which allowed the industrial production of progesterone and other sex hormones from plant steroids.
Sarsasapogenin is unusual in that it has a cis-linkage between rings A and B of the steroid nucleus, as opposed to the more usual trans-linkage found in other saturated steroids. This 5β configuration is biologically significant, as a specific enzyme – sarsasapogenin 3β-glucosyltransferase – is found in several plants for the glycosylation of sarsasapogenin.[4] The (S)-configuration at C-25 is also in contrast to other spirostan sapogenins: the epimer with a (25R)-configuration is known as smilagenin.
Sarsasapogenin has been used as a starting material for the synthesis of other steroids.[5] It has also attracted pharmaceutical interest in its own right,[6][7][8] and is found in the rhizome of Anemarrhena asphodeloides, used in Chinese tradition medicine (知母, zhī mǔ), from which it is extracted commercially.[9]
Occurrence and isolation
Sarsasapogenin is found as a glycoside – with one or more sugar units attached to the hydroxyl group, known as a saponin – in the roots of many species of monocotyledonous plant, in particular:[7]
- Smilax sp.
- Smilax regelii Kilip & C. V. Morton (Honduran sarsapilla)
- Smilax ornata Hook.f. (Jamaican sarsapilla, synonym of S. regelii)
- Smilax aristolochiaefolia Mill. (American sarsapilla)
- Smilax aspera L. (Spanish sarsapilla)
- Smilax glabra Roxb. (in Chinese, tǔfúlíng 土茯苓)
- Smilax febrifuga Kunth (Ecuadorian or Peruvian sarsapilla)
- Smilax regelii Kilip & C. V. Morton (Honduran sarsapilla)
- Asparagus sp.
- Anemarrhena sp.
- Anemarrhena asphodeloides Bunge(in Chinese, zhī mǔ 知母)
- Yucca sp.
- Yucca schidigera Roezl ex Ortges (Mojave yucca)
- Yucca brevifolia Enulm. (Joshua tree)
- Agave sp.
The sarsasapogenin saponin can be extracted from the dried powdered root with 95% ethanol. After removal of the fat from the resulting gum, the glycosidic linkage is hydrolyzed with hydrochloric acid (approx. 2 M) and the resulting crude steroid is recrystallized from anhydrous acetone. The yield of pure sarsasapogenin from 225 kg of Smilax root is reported to be about 450 grams.[1]
History
Sarsasapogenin was first isolated in 1914 from Sarsapilla root.[2] Although it was known to have three oxygen atoms, of which only one is a hydroxyl group, the structure of the side chain remained unclear for many years. Tschesche and Hagedorn proposed an unreactive double tetrahydrofuran structure based on degradation studies which indicated an ether oxygen atom attached to C-16.[10] The true nature of the side chain – a ketone spiro acetal – was discovered by Russell Marker in 1939, when he succeeded in opening the six-membered pyran ring with acetic anhydride.[3] Marker found that almost the entire side chain could be cleaved in three steps, a process now known as the Marker degradation.
Marker was able to convert sarsasapogenin into pregane-3,20-diol[11] (a progesterone analogue) and testosterone.[12] However, for large scale production of steroid hormones, it proved more convenient to use diosgenin (extracted from the Mexican yam Dioscorea mexicana) as the starting material, as it contains a double bond in the steroid nucleus.[13]
Pharmacological interest
Sarsasapogenin and its C-25 epimer smilagenin lowered blood sugar and reversed diabetic weight gain in experiments with in mice with a mutant diabetes gene (db).[6] Both steroids also halted the decline in muscarinic acetylcholine receptors (mAChRs) in animal models of Alzheimer's disease.[7][8] In both cases, the effects seem to be specific to the 5β-configuration, the cis-linkage between rings A and B, as diosgenin (with a Δ5 double bond which can be hydrogenated in the body) had much lower anti-diabetic activity[6] (and no significant effect on mAChRs[7]) while tigogenin (the 5α-epimer of smilagenin) showed no effect at all in either study.[6][7]
References
- ↑ 1.0 1.1 Jacobs, Walter A.; Simpson, James C. E. On Sarsasapogenin and Gitogenin. J. Biol. Chem. 1934, 105 (3), 501–10, <http://www.jbc.org/content/105/3/501.full.pdf>.
- ↑ 2.0 2.1 2.2 Power, Frederick Belding; Salway, Arthur Henry Chemical examination of sarsaparilla root. J. Chem. Soc., Trans. 1914, 105, 201–19. DOI: 10.1039/CT9140500201.
- ↑ 3.0 3.1 Marker, Russell E.; Rohrmann, Ewald Sterols. LIII. The Structure of the Side Chain of Sarsasapogenin. J. Am. Chem. Soc. 1939, 61 (4), 846–51. DOI: 10.1021/ja01873a020.
- ↑ Paczkowski, Cezary; Wojciechowski, Zdzisław A. The occurrence of UDPG-dependent glucosyltransferase specific for sarsasapogenin in Asparagus officinalis. Phytochemistry 1988, 27 (9), 2743–47. DOI: 10.1016/0031-9422(88)80654-X.
- ↑ Dryden, Jr., Hugh L.; Markos, Charles S. (Searle & Co.) Process for the preparation of 17β-hydroxy-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone. US Patent 4057543, published 8 November 1977.
- ↑ 6.0 6.1 6.2 6.3 Applezweig, Norman (Progenics Inc.) Treatment of obesity and diabetes using sapogenins. US Patent 4680289, published 14 July 1987.
- ↑ 7.0 7.1 7.2 7.3 7.4 Xia, Zongqin; Hu, Yaer; Rubin, Ian, et al. (Phytopharm plc) Steroidal sapogenins and their derivatives for treating alzheimer's disease. US Patent 6812213, published 19 December 2002.
- ↑ 8.0 8.1 Hu, Yaer; Xia, Zongqin; Sun, Qixiang; Orsi, Antonia; Rees, Daryl A new approach to the pharmacological regulation of memory: Sarsasapogenin improves memory by elevating the low muscarinic acetylcholine receptor density in brains of memory-deficit rat models. Brain Research 2005, 1060 (1–2), 26–39. DOI: 10.1016/j.brainres.2005.08.019.
- ↑ Sarsasapogenin; Wilshire Technologies, <http://www.wilshiretechnologies.com/steroidal_saponins_and_sapogenins_pdf/sarsasapogenin.pdf>. (accessed 7 March 2010).
- ↑ Tschesche, R.; Hagedorn, A. Über neutrale Saponine, II. Mitteil.: Abbau eines Genins der neutralen Sapogenine zu einem Gallensäure-Derivat. Ber. Dtsch. Chem. Ges. 1935, 68, 1412–20. DOI: 10.1002/cber.19350680736.
- ↑ Marker, Russell E.; Rohrmann, Ewald Sterols. LXXXI. Conversion of Sarsasapogenin to Pregnanediol-3(α),20(α). J. Am. Chem. Soc. 1939, 61. DOI: 10.1021/ja01267a513. Marker, Russell E.; Rohrmann, Ewald Sterols. LXXXVIII. Pregnanediols from Sarsasapogenin. J. Am. Chem. Soc. 1940, 62 (3), 518–20. DOI: 10.1021/ja01860a017.
- ↑ Marker, Russell E. Sterols. CV. The Preparation of Testosterone and Related Compounds from Sarsasapogenin and Diosgenin. J. Am. Chem. Soc. 1940, 62 (9), 2543–47. DOI: 10.1021/ja01866a077.
- ↑ Marker, Russell E.; Tsukamoto, Takeo; Turner, D. L. Sterols. C. Diosgenin. J. Am. Chem. Soc. 1940, 62 (9), 2525–32. DOI: 10.1021/ja01866a072.
| Error creating thumbnail: Unable to save thumbnail to destination |
This page is currently licensed under the Creative Commons Attribution 3.0 Unported license and any later versions of that license. |