Difference between revisions of "File:Sherburn's Total Synthesis of (-)-Artigenin.png"
(Added InChI) |
(Radical Carboxyarylation link) |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 12: | Line 12: | ||
(IV)The terminal alcohol from structure [5] reacts with the C-Cl bond from structure [8] to produce a combined product [9] | (IV)The terminal alcohol from structure [5] reacts with the C-Cl bond from structure [8] to produce a combined product [9] | ||
| − | (V) | + | (V) The conversion from [9] into [10] requires a [[radical carboxyarylation]]. |
| − | (VI) | + | (VI) The TBDMS protecting groups are then removed [11]. |
| − | (VII) | + | (VII) The secondary alcohol is selectively removed to yield our target product (-)-Artigenin [12]. |
| − | |||
| Line 42: | Line 41: | ||
[9]InChI=1/C33H52O5SSi2/c1-15-23(30(41(13,14)33(5,6)7)24-16-18-26(34-8)27(20-24)35-9)22-37-31(39)38-25-17-19-29(28(21-25)36-10)40(11,12)32(2,3)4/h15-21,23,30H,1,22H2,2-14H3/t23-,30+/m1/s1 | [9]InChI=1/C33H52O5SSi2/c1-15-23(30(41(13,14)33(5,6)7)24-16-18-26(34-8)27(20-24)35-9)22-37-31(39)38-25-17-19-29(28(21-25)36-10)40(11,12)32(2,3)4/h15-21,23,30H,1,22H2,2-14H3/t23-,30+/m1/s1 | ||
| − | [10] | + | [10]InChI=1/C33H52O5Si2/c1-32(2,3)39(10,11)29-17-14-22(19-28(29)37-9)18-24-25(21-38-31(24)34)30(40(12,13)33(4,5)6)23-15-16-26(35-7)27(20-23)36-8/h14-17,19-20,24-25,30H,18,21H2,1-13H3/t24-,25+,30-/m1/s1 |
| − | [11] | + | [11]InChI=1/C21H24O7/c1-25-17-7-5-13(10-19(17)27-3)20(23)15-11-28-21(24)14(15)8-12-4-6-16(22)18(9-12)26-2/h4-7,9-10,14-15,20,22-23H,8,11H2,1-3H3/t14-,15+,20-/m1/s1 |
| − | [12] | + | [12]InChI=1/C21H24O6/c1-24-18-7-5-13(11-20(18)26-3)8-15-12-27-21(23)16(15)9-14-4-6-17(22)19(10-14)25-2/h4-7,10-11,15-16,22H,8-9,12H2,1-3H3/t15-,16+/m0/s1 |
Latest revision as of 13:31, 19 May 2010
Sherburn's Total Synthesis of (-)-Arctigenin
The following synthesis begins at the top left and follows the respective arrows to the bottom left product, (-)-Arctigenin.
(I)The first step involves the formation of the boron enolate of the oxazolidinone [1], which then is reacted with the given aldehyde[2] to produce this first structure [3] in an approximate 92% yield.
(II)In the following two steps the chiral alcohol is protected by the creation of an -OTBS group [4]. After which the oxazolidinone is removed to form a terminal alcohol [5].
(III)Separately the aldehyde [6], is reduced to an alcohol [7], the alcohol is reacted with thiophosgene (CSCl2) to produce a very reactive C-Cl bond in place of the alcohol[8].
(IV)The terminal alcohol from structure [5] reacts with the C-Cl bond from structure [8] to produce a combined product [9]
(V) The conversion from [9] into [10] requires a radical carboxyarylation.
(VI) The TBDMS protecting groups are then removed [11].
(VII) The secondary alcohol is selectively removed to yield our target product (-)-Artigenin [12].
InChI
[1]InChI=1/C9H10O3/c1-11-8-4-3-7(6-10)5-9(8)12-2/h3-6H,1-2H3
[2]InChI=1/C14H15NO3/c1-2-6-13(16)15-12(10-18-14(15)17)9-11-7-4-3-5-8-11/h2-8,12H,9-10H2,1H3/b6-2+/t12-/m0/s1
[3]InChI=1/C23H25NO6/c1-4-18(21(25)16-10-11-19(28-2)20(13-16)29-3)22(26)24-17(14-30-23(24)27)12-15-8-6-5-7-9-15/h4-11,13,17-18,21,25H,1,12,14H2,2-3H3/t17-,18-,21+/m0/s1
[4]InChI=1/C29H39NO5Si/c1-9-23(27(31)30-22(19-35-28(30)32)17-20-13-11-10-12-14-20)26(36(7,8)29(2,3)4)21-15-16-24(33-5)25(18-21)34-6/h9-16,18,22-23,26H,1,17,19H2,2-8H3/t22-,23-,26+/m0/s1
[5]InChI=1/C19H32O3Si/c1-9-14(13-20)18(23(7,8)19(2,3)4)15-10-11-16(21-5)17(12-15)22-6/h9-12,14,18,20H,1,13H2,2-8H3/t14-,18+/m1/s1
[6]InChI=1/C14H22O2Si/c1-14(2,3)17(5,6)13-8-7-11(10-15)9-12(13)16-4/h7-10H,1-6H3
[7]InChI=1/C13H22O2Si/c1-13(2,3)16(5,6)12-8-7-10(14)9-11(12)15-4/h7-9,14H,1-6H3
[8]InChI=1/C14H21ClO2SSi/c1-14(2,3)19(5,6)12-8-7-10(17-13(15)18)9-11(12)16-4/h7-9H,1-6H3
[9]InChI=1/C33H52O5SSi2/c1-15-23(30(41(13,14)33(5,6)7)24-16-18-26(34-8)27(20-24)35-9)22-37-31(39)38-25-17-19-29(28(21-25)36-10)40(11,12)32(2,3)4/h15-21,23,30H,1,22H2,2-14H3/t23-,30+/m1/s1
[10]InChI=1/C33H52O5Si2/c1-32(2,3)39(10,11)29-17-14-22(19-28(29)37-9)18-24-25(21-38-31(24)34)30(40(12,13)33(4,5)6)23-15-16-26(35-7)27(20-23)36-8/h14-17,19-20,24-25,30H,18,21H2,1-13H3/t24-,25+,30-/m1/s1
[11]InChI=1/C21H24O7/c1-25-17-7-5-13(10-19(17)27-3)20(23)15-11-28-21(24)14(15)8-12-4-6-16(22)18(9-12)26-2/h4-7,9-10,14-15,20,22-23H,8,11H2,1-3H3/t14-,15+,20-/m1/s1
[12]InChI=1/C21H24O6/c1-24-18-7-5-13(11-20(18)26-3)8-15-12-27-21(23)16(15)9-14-4-6-17(22)19(10-14)25-2/h4-7,10-11,15-16,22H,8-9,12H2,1-3H3/t15-,16+/m0/s1
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 12:55, 19 May 2010 | 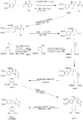 | 2,145 × 3,108 (131 KB) | Terpstra (talk | contribs) | |
| 14:31, 15 May 2010 |  | 504 × 799 (25 KB) | Terpstra (talk | contribs) | Sherburn's Total SYnthesis of (-)-Arctigenin |
- You cannot overwrite this file.
File usage
The following page links to this file: