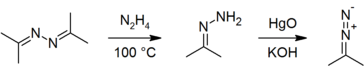Difference between revisions of "Acetone azine"
Physchim62 (talk | contribs) (→Laboratory preparation and use) |
Physchim62 (talk | contribs) |
||
| (9 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{chembox | {{chembox | ||
| + | | Name = Acetone azine | ||
| + | | ImageFile = Acetone_azine.png | ||
| + | | ImageSize = 200px | ||
| + | | ImageName = Acetone azine | ||
| IUPACName = acetone azine<br/>diisopropylidenehydrazine | | IUPACName = acetone azine<br/>diisopropylidenehydrazine | ||
| + | | OtherNames = dimethyl ketazine | ||
| Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| CASNo = 627-70-3 | | CASNo = 627-70-3 | ||
| Line 22: | Line 27: | ||
}} | }} | ||
}} | }} | ||
| − | '''Acetone azine''', Me<sub>2</sub>C=N–N=CMe<sub>2</sub>, is the [[Condensation reaction|condensation product]] of two [[equivalent (chemistry)|equivalent]]s of [[acetone]] with one equivalent of [[hydrazine]]. It is an intermediate in the industrial production of hydrazine by the [[ | + | '''Acetone azine''', Me<sub>2</sub>C=N–N=CMe<sub>2</sub>, is the [[Condensation reaction|condensation product]] of two [[equivalent (chemistry)|equivalent]]s of [[acetone]] with one equivalent of [[hydrazine]]. It is an intermediate in the industrial production of hydrazine by the [[Pechiney-Ugine-Kuhlmann process]]<ref name="PCUK">{{citation | inventor1-first = Jean-Pierre | inventor1-last = Schirmann | inventor2-first = Jean | inventor2-last = Combroux | inventor3-first = Serge Yvon | inventor3-last = Delavarenne | assignee = Produits Chimiques Ugine Kuhlmann | country-code = US | patent-number = 3972878 | title = Method for preparing azines and hydrazones | issue-date = 1976-08-03}}. {{citation | inventor1-first = Jean-Pierre | inventor1-last = Schirmann | inventor2-first = Pierre | inventor2-last = Tellier | inventor3-first = Henri | inventor3-last = Mathais | inventor4-first = Francis | inventor4-last = Weiss | assignee = Produits Chimiques Ugine Kuhlmann | country-code = US | patent-number = 3978049 | title = Process for the preparation of hydrazine compounds | issue-date = 1976-08-31}}. {{citation | inventor1-first = Jean-Pierre | inventor1-last = Schirmann | inventor2-first = Jean | inventor2-last = Combroux | inventor3-first = Serge Yvon | inventor3-last = Delavarenne | assignee = Produits Chimiques Ugine Kuhlmann | country-code = US | patent-number = 4093656 | title = Method for making azines | issue-date = 1978-06-06}}.</ref><ref name="Atochem">{{citation | inventor1-first = Jean-Pierre | inventor1-last = Schirmann | inventor2-first = Jean | inventor2-last = Combroux | inventor3-first = Serge Y. | inventor3-last = Delavarenne | assignee = Atochem | country-code = US | patent-number = 4724133 | title = Preparation of a concentrated aqueous solution of hydrazine hydrate | issue-date = 1988-02-09}}.</ref> and by the [[Bayer hydrazine process]].<ref name="Bayer">{{citation | inventor1-last = Eichenhofer | inventor1-first = Kurt-Wilhelm | inventor2-last = Schliebs | inventor2-first = Reinhard | assignee = Bayer | title = Production of ketazines | country-code = US | patent-number = 3965097 | issue-date = 1976-06-22}}.</ref><ref name="H&W">{{Holleman&Wiberg|page=619}}.</ref> |
==Laboratory preparation and use== | ==Laboratory preparation and use== | ||
| − | On a laboratory scale, acetone azine is prepared by the direct reaction of acetone with [[hydrazine hydrate]], with water being removed from the product by stirring with solid [[potassium hydroxide]]:<ref>{{citation | first1 = T. | last1 = Curtius | first2 = K. | last2 = Thun | journal = J. Prakt. Chem. | volume = 44 | pages = | + | On a laboratory scale, acetone azine is prepared by the direct reaction of acetone with [[hydrazine hydrate]], with water being removed from the product by stirring with solid [[potassium hydroxide]]:<ref>{{citation | first1 = T. | last1 = Curtius | first2 = K. | last2 = Thun | title = Einwirkung von Hydrazinhydrat auf Monoketone und Orthodiketone | journal = J. Prakt. Chem. (2. Ser.) | year = 1891 | volume = 44 | pages = 161–86 | doi = 10.1002/prac.18910440121}}.</ref><ref name="OrgSynth">{{OrgSynth | first1 = A. C. | last1 = Day | first2 = M. C. | last2 = Whiting | title = Acetone hydrazone | collvol = 6 | collvolpages = 10 | volume = 50 | pages =3 | year = 1970 | prep = cv6p0010}}.</ref> it is also commercially available. It is a useful precursor to [[acetone hydrazone]]<ref name="OrgSynth"/><ref name="Ber">{{citation | first1 = H. | last1 = Staudinger | first2 = Alice | last2 = Gaule | title = Vergleich der Stickstoff-Abspaltung bei verschiedenen aliphatischen Diazoverbindungen | journal = Ber. Dtsch. Chem. Ges. | year = 1916 | volume = 49 | issue = 2 | pages = 1897–1918 | doi = 10.1002/cber.19160490245}}.</ref> and hence to [[2-diazopropane]],<ref name="Ber"/><ref>{{citation | first1 = A. C. | last1 = Day | first2 = P. | last2 = Raymond | first3 = R. M. | last3 = Southam | first4 = M. C. | last4 = Whiting | title = The preparation of secondary aliphatic diazo-compounds from hydrazones | journal = J. Chem. Soc. C | year = 1966 | pages = 467–69 | doi = 10.1039/J39660000467}}.</ref><ref name="diazo">{{OrgSynth | first1 = S. D. | last1 = Andrews | first2 = A. C. | last2 = Day | first3 = P. | last3 = Raymond | first4 = M. C. | last4 = Whiting | title = 2-Diazopropane | collvol = 6 | collvolpages = 392 | volume = 50 | pages = 27 | year = 1970 | prep = cv6p0392}}.</ref> both of which must be prepared immediately before use and cannot be stored.<ref name="OrgSynth"/><ref name="diazo"/> |
| − | As well as its use as an intermediate in [[organic synthesis]], the [[coordination chemistry]] of acetone azine (as a [[ligand]]) has also been studied.<ref>{{citation | first1 = A. S. | last1 = Gudkova | first2 = O. A. | last2 = Reutov | first3 = M. Ya. | last3 = Aleinikova | journal = Izv. Akad. Nauk SSSR, Otdel. Khim. Nauk | year = 1962 | issue = 8 | pages = 1382–87}}; {{citation | title = Reactions of hydrazones and azines with metal salts 4. Reactions of azines of aldehydes and ketones with cupric salts | journal = Russ. Chem. Bull. (Transl.) | year = 1962 | volume = 11 | issue = 8 | pages = 1298–1302 | doi = 10.1007/BF00907973}}.</ref><ref>{{citation | first1 = A. S. | last1 = Gudkova | first2 = M. Ya. | last2 = Aleinikova | first3 = O. A. | last3 = Reutov | journal = Izv. Akad. Nauk SSSR, Ser. Khim. | issue = 5 | pages = 844–48 | year = 1966}}; {{citation | title = Reactions of hydrazones and azines with metal salts 5. Reactions of hydrazones and azines with mercuric halides | journal = Russ. Chem. Bull. (Transl.) | volume = 15 | issue = 5 | year = 1966 | pages = 807–11 | doi = 10.1007/BF00849376}}.</ref><ref>{{citation | first1 = Fiona | last1 = King | first2 = David | last2 = Nicholls | title = Complex of titanium halides with acetone azine and its isomer 3,5,5-trimethyl-pyrazoline | journal = Inorg. Chim. Acta | volume = 28 | year = 1978 | pages = 55–58 | doi = 10.1016/S0020-1693(00)87413-7}}.</ref> | + | [[File:Acetone azine chem.png|364px]] |
| + | |||
| + | As well as its use as an intermediate in [[organic synthesis]], the [[coordination chemistry]] of acetone azine (as a [[ligand]]) has also been studied.<ref>{{citation | first1 = A. S. | last1 = Gudkova | first2 = O. A. | last2 = Reutov | first3 = M. Ya. | last3 = Aleinikova | journal = Izv. Akad. Nauk SSSR, Otdel. Khim. Nauk | year = 1962 | issue = 8 | pages = 1382–87}}; {{citation | title = Reactions of hydrazones and azines with metal salts 4. Reactions of azines of aldehydes and ketones with cupric salts | journal = Russ. Chem. Bull. (Transl.) | year = 1962 | volume = 11 | issue = 8 | pages = 1298–1302 | doi = 10.1007/BF00907973}}.</ref><ref>{{citation | first1 = A. S. | last1 = Gudkova | first2 = M. Ya. | last2 = Aleinikova | first3 = O. A. | last3 = Reutov | journal = Izv. Akad. Nauk SSSR, Ser. Khim. | issue = 5 | pages = 844–48 | year = 1966}}; {{citation | title = Reactions of hydrazones and azines with metal salts 5. Reactions of hydrazones and azines with mercuric halides | journal = Russ. Chem. Bull. (Transl.) | volume = 15 | issue = 5 | year = 1966 | pages = 807–11 | doi = 10.1007/BF00849376}}.</ref><ref>{{citation | first1 = Fiona | last1 = King | first2 = David | last2 = Nicholls | title = Complex of titanium halides with acetone azine and its isomer 3,5,5-trimethyl-pyrazoline | journal = Inorg. Chim. Acta | volume = 28 | year = 1978 | pages = 55–58 | doi = 10.1016/S0020-1693(00)87413-7}}.</ref> Acetone is also used to derivatize hydrazine, through formation of acetone azine, for analysis by [[gas chromatography]]: the method has been used to determine trace levels of hydrazine in drinking water<ref>{{citation | journal = Anal. Chem. | year = 2008 | volume = 80 | issue = 14 | pages = 5449–53 | title = Analysis of hydrazine in drinking water by isotope dilution gas chromatography/tandem mass spectrometry with derivatization and liquid-liquid extraction | last1 = Davis | first1 = William E., II | last2 = Li | first2 = Yongtao | doi = 10.1021/ac702536d}}.</ref> and pharmaceuticals.<ref>{{citation | journal = J. Pharm. Biomed. Anal. | year = 2009 | volume = 49 | issue = 2 | pages = 529–33 | title = A generic approach for the determination of trace hydrazine in drug substances using ''in situ'' derivatization-headspace GC–MS | last1 = Sun | first1 = Mingjiang | last2 = Bai | first2 = Lin | last3 = Liu | first3 = David Q. | doi = 10.1016/j.jpba.2008.11.009}}.</ref> | ||
==Production of hydrazine== | ==Production of hydrazine== | ||
| − | === | + | ===Pechiney-Ugine-Kuhlmann process=== |
The industrial preparation of acetone azine<ref name="PCUK"/> might, at first sight, appear to be the reverse of the laboratory procedure: the azine is produced from [[acetone hydrazone]], and then [[Hydrolysis|hydrolyzed]] to give [[hydrazine hydrate]] and [[acetone]]. The interest in preparing the azine is that it can be removed from the initial reaction mixture as an [[azeotrope]] with water:<ref name="Atochem"/> in both laboratory practice and the Atofina–PCUK process, the aim is to avoid directly handling the unstable acetone hydrazone. | The industrial preparation of acetone azine<ref name="PCUK"/> might, at first sight, appear to be the reverse of the laboratory procedure: the azine is produced from [[acetone hydrazone]], and then [[Hydrolysis|hydrolyzed]] to give [[hydrazine hydrate]] and [[acetone]]. The interest in preparing the azine is that it can be removed from the initial reaction mixture as an [[azeotrope]] with water:<ref name="Atochem"/> in both laboratory practice and the Atofina–PCUK process, the aim is to avoid directly handling the unstable acetone hydrazone. | ||
| − | |||
| − | |||
| − | |||
| − | |||
===Bayer hydrazine process=== | ===Bayer hydrazine process=== | ||
| − | The [[Bayer hydrazine process]] is a modification of the [[Raschig | + | The [[Bayer hydrazine process]] is a modification of the [[Raschig process]], that is the oxidation of [[ammonia]] with [[sodium hypochlorite]] to produce [[hydrazine]]. The addition of [[acetone]] to the reaction mixture allows the separation and purification of acetone azine before its [[hydrolysis]] to [[hydrazine hydrate]].<ref name="H&W"/> |
==References== | ==References== | ||
Latest revision as of 15:24, 2 July 2010
| Acetone azine | |
|---|---|

| |
| IUPAC name | acetone azine diisopropylidenehydrazine |
| Other names | dimethyl ketazine |
| Identifiers | |
| InChI | InChI=1/C6H12N2/c1-5(2)7-8-6(3)4/h1-4H3 |
| InChIKey | PFLUPZGCTVGDLV-UHFFFAOYAR |
| Standard InChI | InChI=1S/C6H12N2/c1-5(2)7-8-6(3)4/h1-4H3 |
| Standard InChIKey | PFLUPZGCTVGDLV-UHFFFAOYSA-N |
| CAS number | [] |
| EC number | |
| ChemSpider | |
| SMILES | |
| Properties[1] | |
| Chemical formula | C6H12N2 |
| Molar mass | 112.17 g mol−1 |
| Appearance | colourless liquid |
| Density | 0.8390 g cm−3 |
| Melting point |
−12.5 °C |
| Boiling point |
133 °C |
| Refractive index (nD) | 1.4535 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Acetone azine, Me2C=N–N=CMe2, is the condensation product of two equivalents of acetone with one equivalent of hydrazine. It is an intermediate in the industrial production of hydrazine by the Pechiney-Ugine-Kuhlmann process[2][3] and by the Bayer hydrazine process.[4][5]
Contents
Laboratory preparation and use
On a laboratory scale, acetone azine is prepared by the direct reaction of acetone with hydrazine hydrate, with water being removed from the product by stirring with solid potassium hydroxide:[6][7] it is also commercially available. It is a useful precursor to acetone hydrazone[7][8] and hence to 2-diazopropane,[8][9][10] both of which must be prepared immediately before use and cannot be stored.[7][10]
As well as its use as an intermediate in organic synthesis, the coordination chemistry of acetone azine (as a ligand) has also been studied.[11][12][13] Acetone is also used to derivatize hydrazine, through formation of acetone azine, for analysis by gas chromatography: the method has been used to determine trace levels of hydrazine in drinking water[14] and pharmaceuticals.[15]
Production of hydrazine
Pechiney-Ugine-Kuhlmann process
The industrial preparation of acetone azine[2] might, at first sight, appear to be the reverse of the laboratory procedure: the azine is produced from acetone hydrazone, and then hydrolyzed to give hydrazine hydrate and acetone. The interest in preparing the azine is that it can be removed from the initial reaction mixture as an azeotrope with water:[3] in both laboratory practice and the Atofina–PCUK process, the aim is to avoid directly handling the unstable acetone hydrazone.
Bayer hydrazine process
The Bayer hydrazine process is a modification of the Raschig process, that is the oxidation of ammonia with sodium hypochlorite to produce hydrazine. The addition of acetone to the reaction mixture allows the separation and purification of acetone azine before its hydrolysis to hydrazine hydrate.[5]
References
- ↑ CRC Handbook of Chemistry and Physics, 62nd ed.; Weast, Robert C., Ed.; CRC Press: Boca Raton, FL, 1981; p C-74. ISBN 0-8493-0462-8.
- ↑ 2.0 2.1 Schirmann, Jean-Pierre; Combroux, Jean; Delavarenne, Serge Yvon (Produits Chimiques Ugine Kuhlmann) Method for preparing azines and hydrazones. US Patent 3972878, issued 3 August 1976. Schirmann, Jean-Pierre; Tellier, Pierre; Mathais, Henri, et al. (Produits Chimiques Ugine Kuhlmann) Process for the preparation of hydrazine compounds. US Patent 3978049, issued 31 August 1976. Schirmann, Jean-Pierre; Combroux, Jean; Delavarenne, Serge Yvon (Produits Chimiques Ugine Kuhlmann) Method for making azines. US Patent 4093656, issued 6 June 1978.
- ↑ 3.0 3.1 Schirmann, Jean-Pierre; Combroux, Jean; Delavarenne, Serge Y. (Atochem) Preparation of a concentrated aqueous solution of hydrazine hydrate. US Patent 4724133, issued 9 February 1988.
- ↑ Eichenhofer, Kurt-Wilhelm; Schliebs, Reinhard (Bayer) Production of ketazines. US Patent 3965097, issued 22 June 1976.
- ↑ 5.0 5.1 Holleman, A. F.; Wiberg, E. Inorganic Chemistry; Academic Press: San Diego, 2001; p 619. ISBN 0-12-352651-5.
- ↑ Curtius, T.; Thun, K. Einwirkung von Hydrazinhydrat auf Monoketone und Orthodiketone. J. Prakt. Chem. (2. Ser.) 1891, 44, 161–86. DOI: 10.1002/prac.18910440121.
- ↑ 7.0 7.1 7.2 Day, A. C.; Whiting, M. C. Acetone hydrazone. Org. Synth. 1970, 50, 3, <http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0010>; Coll. Vol., 6, 10.
- ↑ 8.0 8.1 Staudinger, H.; Gaule, Alice Vergleich der Stickstoff-Abspaltung bei verschiedenen aliphatischen Diazoverbindungen. Ber. Dtsch. Chem. Ges. 1916, 49 (2), 1897–1918. DOI: 10.1002/cber.19160490245.
- ↑ Day, A. C.; Raymond, P.; Southam, R. M.; Whiting, M. C. The preparation of secondary aliphatic diazo-compounds from hydrazones. J. Chem. Soc. C 1966, 467–69. DOI: 10.1039/J39660000467.
- ↑ 10.0 10.1 Andrews, S. D.; Day, A. C.; Raymond, P.; Whiting, M. C. 2-Diazopropane. Org. Synth. 1970, 50, 27, <http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0392>; Coll. Vol., 6, 392.
- ↑ Gudkova, A. S.; Reutov, O. A.; Aleinikova, M. Ya. Izv. Akad. Nauk SSSR, Otdel. Khim. Nauk 1962 (8), 1382–87; Reactions of hydrazones and azines with metal salts 4. Reactions of azines of aldehydes and ketones with cupric salts. Russ. Chem. Bull. (Transl.) 1962, 11 (8), 1298–1302. DOI: 10.1007/BF00907973.
- ↑ Gudkova, A. S.; Aleinikova, M. Ya.; Reutov, O. A. Izv. Akad. Nauk SSSR, Ser. Khim. 1966 (5), 844–48; Reactions of hydrazones and azines with metal salts 5. Reactions of hydrazones and azines with mercuric halides. Russ. Chem. Bull. (Transl.) 1966, 15 (5), 807–11. DOI: 10.1007/BF00849376.
- ↑ King, Fiona; Nicholls, David Complex of titanium halides with acetone azine and its isomer 3,5,5-trimethyl-pyrazoline. Inorg. Chim. Acta 1978, 28, 55–58. DOI: 10.1016/S0020-1693(00)87413-7.
- ↑ Davis, William E., II; Li, Yongtao Analysis of hydrazine in drinking water by isotope dilution gas chromatography/tandem mass spectrometry with derivatization and liquid-liquid extraction. Anal. Chem. 2008, 80 (14), 5449–53. DOI: 10.1021/ac702536d.
- ↑ Sun, Mingjiang; Bai, Lin; Liu, David Q. A generic approach for the determination of trace hydrazine in drug substances using in situ derivatization-headspace GC–MS. J. Pharm. Biomed. Anal. 2009, 49 (2), 529–33. DOI: 10.1016/j.jpba.2008.11.009.
| Error creating thumbnail: Unable to save thumbnail to destination |
This page is currently licensed under the Creative Commons Attribution 3.0 Unported license and any later versions of that license. |