Difference between revisions of "Substance:Ethyl acetate"
(Create) |
(Fix for image) |
||
| Line 1: | Line 1: | ||
{| class="wikitable" border="0" style="float: right;" | {| class="wikitable" border="0" style="float: right;" | ||
|- | |- | ||
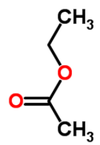
| − | |<!--column1-->[[File: | + | |<!--column1-->[[File:EthylAcetate.png|100px|Structure of ethyl acetate]] |
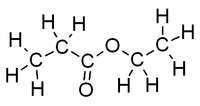
|<!--column2-->[[File:EthylAcetateFullStructure.png|200px|Structure of ethyl acetate with all atoms shown]] | |<!--column2-->[[File:EthylAcetateFullStructure.png|200px|Structure of ethyl acetate with all atoms shown]] | ||
|}<!--end wikitable--> | |}<!--end wikitable--> | ||
Revision as of 00:20, 10 September 2010
|
|
- Main ChemSpider page
- Molecular formula: C4H8O2
- Molar mass: 88.1051
- CAS Registry Number: 141-78-6
- IUPAC name: Ethyl acetate
- Appearance: Colorless liquid with an ether-like, fruity odor.
- Boiling point: 77 °C
REMAINDER NEED FIXING
- Solubility properties: Not available
- Safety sheet: From ChemAdvisor
- Spectra: IR, 1H NMR, 13C NMR, MS. Also check on SDBS.
From Wikipedia
Ethyl acetate (systematically, ethyl ethanoate, commonly abbreviated EtOAc or EA) is the organic compound with the formula CH3COOCH2CH3. This colorless liquid has a characteristic sweet smell (similar to pear drops) like certain glues or nail polish removers, in which it is used. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent. In 1985, about 400,000 tons were produced yearly in Japan, North America, and Europe combined. In 2004, an estimated 1.3M tons were produced worldwide. Read more... or edit at Wikipedia.