Difference between revisions of "Peroxydisulfuric acid"
Physchim62 (talk | contribs) (Imported from http://en.wikipedia.org/w/index.php?title=Peroxydisulfuric_acid&oldid=296961758) |
Physchim62 (talk | contribs) (refs) |
||
| Line 10: | Line 10: | ||
| PubChem = 24413 | | PubChem = 24413 | ||
| ChEBI = 29268 | | ChEBI = 29268 | ||
| − | | InChI = 1/H2O8S2/c1-9(2,3)7-8-10(4,5)6/h(H,1,2,3)(H,4,5,6)/ | + | | InChI = 1/H2O8S2/c1-9(2,3)7-8-10(4,5)6/h(H,1,2,3)(H,4,5,6) |
| + | | StdInChI = 1S/H2O8S2/c1-9(2,3)7-8-10(4,5)6/h(H,1,2,3)(H,4,5,6) | ||
| + | | InChIKey = JRKICGRDRMAZLK-UHFFFAOYAS | ||
| + | | StdInChIKey = JRKICGRDRMAZLK-UHFFFAOYSA-N | ||
| SMILES = OS(=O)(=O)OOS(=O)(=O)O | | SMILES = OS(=O)(=O)OOS(=O)(=O)O | ||
| − | }} | + | }} |
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| + | | Reference = <ref name="G&E">{{Greenwood&Earnshaw1st|page=846}}</ref> | ||
| H = 2 | S = 2 | O = 8 | | H = 2 | S = 2 | O = 8 | ||
| − | | Appearance = | + | | Appearance = colourless solid |
| Density = | | Density = | ||
| MeltingPtC = 65 | | MeltingPtC = 65 | ||
| − | | Melting_notes = | + | | Melting_notes = decomp. |
| BoilingPt = | | BoilingPt = | ||
| − | | Solubility = | + | | Solubility = very soluble |
| − | }} | + | }} |
| Section7 = {{Chembox Related | | Section7 = {{Chembox Related | ||
| − | | OtherCpds = [[Potassium | + | | OtherCpds = [[Potassium persulfate]] |
| − | }} | + | }} |
}} | }} | ||
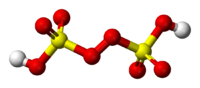
| − | '''Peroxydisulfuric ''' is a [[sulfur]] [[oxoacid]] with the [[chemical formula]] H<sub>2</sub>S<sub>2</sub>O<sub>8</sub> | + | '''Peroxydisulfuric ''' is a [[sulfur]] [[oxoacid]] with the [[chemical formula]] H<sub>2</sub>S<sub>2</sub>O<sub>8</sub>. In structural terms it can be written HO<sub>3</sub>SOOSO<sub>3</sub>H.<ref name="G&E"/> It is one of a group of [[sulfur oxoacids]], its salts, commonly known as persulfates, are industrially important but the acid itself is not. The salts contain the [[peroxydisulfate]] ion, S<sub>2</sub>O<sub>8</sub><sup>2−</sup>, with O–O = 131 pm and S–O<sub>µ</sub> = 150 pm.<ref name="G&E"/> |
| − | . In structural terms it can be written HO<sub>3</sub>SOOSO<sub>3</sub>H. It is one of a group of sulfur oxoacids, its salts, commonly known as persulfates, are industrially important but the acid itself is not. The salts contain the [[ | ||
| − | |||
| − | == | ||
| − | |||
== References == | == References == | ||
Revision as of 12:14, 20 August 2009
| Peroxydisulfuric acid | |
|---|---|

| |
| |
| IUPAC name | μ-peroxido-bis(hydroxidodioxidosulfur) peroxydisulfuric acid |
| Other names | Persulfuric acid |
| Identifiers | |
| InChI | InChI=1/H2O8S2/c1-9(2,3)7-8-10(4,5)6/h(H,1,2,3)(H,4,5,6) |
| InChIKey | JRKICGRDRMAZLK-UHFFFAOYAS |
| Standard InChI | InChI=1S/H2O8S2/c1-9(2,3)7-8-10(4,5)6/h(H,1,2,3)(H,4,5,6) |
| Standard InChIKey | JRKICGRDRMAZLK-UHFFFAOYSA-N |
| CAS number | [] |
| PubChem | |
| ChEBI | 29268 |
| SMILES | |
| Properties[1] | |
| Molecular formula | H2O8S2 |
| Molar mass | 194.14 g mol−1 |
| Appearance | colourless solid |
| Melting point |
65 °C, 338 K, 149 °F decomp. |
| Solubility in water | very soluble |
| Related compounds | |
| Other compounds | Potassium persulfate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Peroxydisulfuric is a sulfur oxoacid with the chemical formula H2S2O8. In structural terms it can be written HO3SOOSO3H.[1] It is one of a group of sulfur oxoacids, its salts, commonly known as persulfates, are industrially important but the acid itself is not. The salts contain the peroxydisulfate ion, S2O82−, with O–O = 131 pm and S–Oµ = 150 pm.[1]
References
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Peroxydisulfuric acid". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |