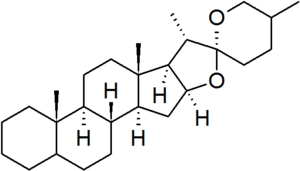Difference between revisions of "Spirostan"
Physchim62 (talk | contribs) |
Physchim62 (talk | contribs) |
||
| Line 6: | Line 6: | ||
! C-5 | ! C-5 | ||
! C-25 | ! C-25 | ||
| + | ! Other | ||
|- | |- | ||
| [[sarsasapogenin]] | | [[sarsasapogenin]] | ||
| Line 19: | Line 20: | ||
| align=center | ''R'' | | align=center | ''R'' | ||
|- | |- | ||
| − | | [[ | + | | [[diosgenin]] |
| align=center | Δ<sup>5</sup> | | align=center | Δ<sup>5</sup> | ||
| align=center | ''R'' | | align=center | ''R'' | ||
| + | |- | ||
| + | | [[anzurogenin-D]] | ||
| + | | α | ||
| + | | ''R'' | ||
| + | | 3β-OH, 5α-OH, 6β-OH | ||
| + | |- | ||
| + | | [[sisalgenin]] | ||
| + | | α | ||
| + | | ''S'' | ||
| + | | C=O at C-12 | ||
| + | |- | ||
| + | | [[roscogenin]] | ||
| + | | Δ<sup>5</sup> | ||
| + | | ''S'' | ||
| + | | | ||
|- | |- | ||
|} | |} | ||
Revision as of 16:24, 8 March 2010
Spirostan is a defined parent hydride in the nomenclature of steroids.[1] It is characterised by a bicyclic side chain containing a ketone spiro acetal group. In the spirostan structure, the configurations of carbons 5 and 25 is not defined, and so must be specified for each derivative. Spirostan steroids include:
| C-5 | C-25 | Other | |
|---|---|---|---|
| sarsasapogenin | β | S | |
| smilagenin | β | R | |
| tigogenin | α | R | |
| diosgenin | Δ5 | R | |
| anzurogenin-D | α | R | 3β-OH, 5α-OH, 6β-OH |
| sisalgenin | α | S | C=O at C-12 |
| roscogenin | Δ5 | S |
References
- ↑ Revised Section F: Natural Products and Related Compounds (IUPAC Recommendations 1999). Pure Appl. Chem., 71 (4), 587–643. DOI: 10.1351/pac199971040587.