Difference between revisions of "Nomenclature of steroids"
Physchim62 (talk | contribs) (→Parent hydride names) |
Physchim62 (talk | contribs) |
||
| Line 1: | Line 1: | ||
| − | The '''nomenclature of steroids''' is a subset of the [[nomenclature of natural products]].<ref name="NatProds">{{IUPAC natural products 1999}}.</ref> | + | The '''nomenclature of steroids''' is a subset of the [[nomenclature of natural products]].<ref name="NatProds">{{IUPAC natural products 1999}}.</ref><ref>{{IUPAC-IUB steroids 1989}}.</ref> |
==Definitions== | ==Definitions== | ||
Revision as of 09:35, 9 March 2010
The nomenclature of steroids is a subset of the nomenclature of natural products.[1][2]
Definitions
A steroid is a compound (either naturally occurring or artificial) based on the cyclopenta[a]phenanthrene carbon skeleton, partially or completely hydrogenated.[3][4] Steroids usually have methyl groups at C-10 and C-13, and often an alkyl group at C-17 (termed a "side chain"). By extension, one or more bond scissions, ring expansions and/or ring contractions of the skeleton may have occurred.[3][4] Sterols are steroids with a hydroxyl group at C-3.[4]
Fundamental parent structures
| Name | Structure | Notes |
|---|---|---|
| gonane | 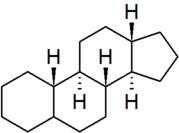
|
|
| estrane | 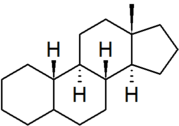
|
"estrane" is the IUPAC preferred spelling; older British texts may spell it as "oestrane" |
| androstane | 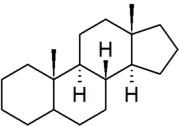
|
|
| pregnane | 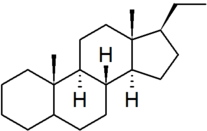
|
|
| cholane | 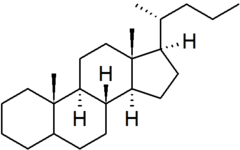
|
|
| cholestane | 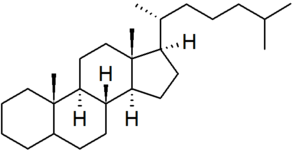
|
|
| ergostane | 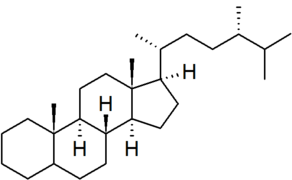
|
|
| campestane | 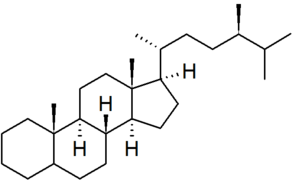
|
|
| poriferastane | 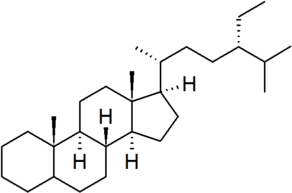
|
|
| stigmastane | 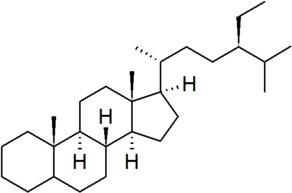
|
|
| gorgostane | 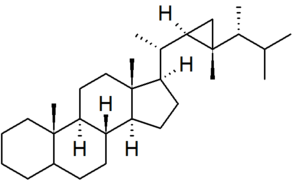
|
numbering of the side chain is non-systematic in CAS nomenclature |
| cardanolide | 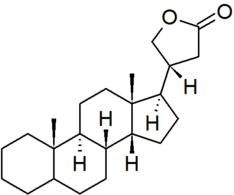
|
|
| bufanolide | 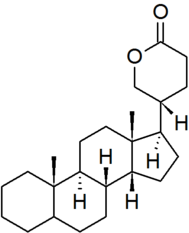
|
|
| furostan | 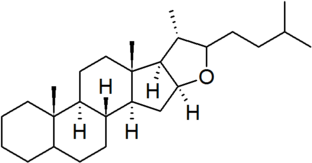
|
configuration at C-22 must be specified for each derivative |
| spirostan | 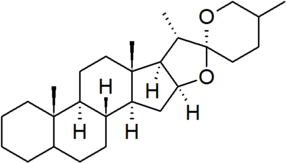
|
configuration at C-25 must be specified for each derivative |
References
- ↑ Revised Section F: Natural Products and Related Compounds (IUPAC Recommendations 1999). Pure Appl. Chem., 71 (4), 587–643. DOI: 10.1351/pac199971040587.
- ↑ Nomenclature of Steroids (IUPAC–IUB Recommendations 1989). Pure Appl. Chem., 61 (10), 1783–1822. DOI: 10.1351/pac198961101783.
- ↑ 3.0 3.1 steroids, <http://goldbook.iupac.org/S06005.html> (accessed 8 March 2010), Compendium of Chemical Terminology Internet edition; International Union of Pure and Applied Chemistry (IUPAC).
- ↑ 4.0 4.1 4.2 Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67 (8-9), 1307–75 at 1367. DOI: 10.1351/pac199567081307.
| Error creating thumbnail: Unable to save thumbnail to destination |
This page is currently licensed under the Creative Commons Attribution 3.0 Unported license and any later versions of that license. |