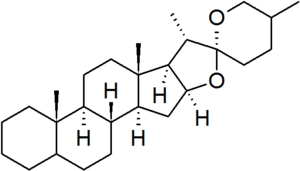Difference between revisions of "Spirostan"
Physchim62 (talk | contribs) |
Physchim62 (talk | contribs) |
||
| Line 1: | Line 1: | ||
[[File:Spirostan.png|thumb|right]] | [[File:Spirostan.png|thumb|right]] | ||
| − | '''Spirostan''' is a defined [[parent hydride]] in the [[nomenclature of steroids]].<ref>{{IUPAC natural products 1999}}.</ref> It is characterised by a bicyclic side chain containing a [[ketone]] [[spiro]] [[acetal]] group. In the spirostan structure, the configurations of carbons 5 and 25 is not defined, and so must be specified for each derivative. Spirostan steroids include: | + | '''Spirostan''' is a defined [[parent hydride]] in the [[nomenclature of steroids]].<ref>{{IUPAC natural products 1999}}.</ref> It is characterised by a bicyclic side chain containing a [[ketone]] [[spiro]] [[acetal]] group. In the spirostan structure, the configurations of carbons 5 and 25 is not defined, and so must be specified for each derivative. Spirostan steroids are common as the [[Glycoside|aglycosidic]] portions of plant [[saponin]]s, and include: |
| + | |||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 11: | Line 12: | ||
| align=center | β | | align=center | β | ||
| align=center | ''S'' | | align=center | ''S'' | ||
| + | | align=center | 3β-OH | ||
|- | |- | ||
| [[smilagenin]] | | [[smilagenin]] | ||
| align=center | β | | align=center | β | ||
| align=center | ''R'' | | align=center | ''R'' | ||
| + | | align=center | 3β-OH | ||
|- | |- | ||
| [[tigogenin]] | | [[tigogenin]] | ||
| align=center | α | | align=center | α | ||
| align=center | ''R'' | | align=center | ''R'' | ||
| + | | align=center | 3β-OH | ||
|- | |- | ||
| [[diosgenin]] | | [[diosgenin]] | ||
| align=center | Δ<sup>5</sup> | | align=center | Δ<sup>5</sup> | ||
| align=center | ''R'' | | align=center | ''R'' | ||
| + | | align=center | 3β-OH | ||
|- | |- | ||
| [[anzurogenin-D]] | | [[anzurogenin-D]] | ||
| − | | α | + | | align=center | α |
| − | | ''R'' | + | | align=center | ''R'' |
| − | | 3β-OH, 5α-OH, 6β-OH | + | | align=center | 3β-OH, 5α-OH, 6β-OH |
|- | |- | ||
| [[sisalgenin]] | | [[sisalgenin]] | ||
| − | | α | + | | align=center | α |
| − | | ''S'' | + | | align=center | ''S'' |
| − | | C=O at C-12 | + | | align=center | 3β-OH, C=O at C-12 |
|- | |- | ||
| [[roscogenin]] | | [[roscogenin]] | ||
| − | | Δ<sup>5</sup> | + | | align=center | Δ<sup>5</sup> |
| − | | ''S'' | + | | align=center | ''S'' |
| − | | | + | | align=center | 1β-OH, 3β-OH |
|- | |- | ||
|} | |} | ||
Revision as of 16:36, 8 March 2010
Spirostan is a defined parent hydride in the nomenclature of steroids.[1] It is characterised by a bicyclic side chain containing a ketone spiro acetal group. In the spirostan structure, the configurations of carbons 5 and 25 is not defined, and so must be specified for each derivative. Spirostan steroids are common as the aglycosidic portions of plant saponins, and include:
| C-5 | C-25 | Other | |
|---|---|---|---|
| sarsasapogenin | β | S | 3β-OH |
| smilagenin | β | R | 3β-OH |
| tigogenin | α | R | 3β-OH |
| diosgenin | Δ5 | R | 3β-OH |
| anzurogenin-D | α | R | 3β-OH, 5α-OH, 6β-OH |
| sisalgenin | α | S | 3β-OH, C=O at C-12 |
| roscogenin | Δ5 | S | 1β-OH, 3β-OH |
References
- ↑ Revised Section F: Natural Products and Related Compounds (IUPAC Recommendations 1999). Pure Appl. Chem., 71 (4), 587–643. DOI: 10.1351/pac199971040587.