Difference between revisions of "File:Sherburn's Total Synthesis of (-)-Artigenin.png"
(Synthesis steps described (needs work)) |
|||
| Line 4: | Line 4: | ||
| − | The first step involves the formation of the boron enolate of the oxazolidinone (right structure), which then is reacted with the given aldehyde(left) to produce this first structure in an approximate 92% yield. | + | [1]The first step involves the formation of the boron enolate of the oxazolidinone (right structure), which then is reacted with the given aldehyde(left) to produce this first structure in an approximate 92% yield. |
| − | In the following two steps the chiral alcohol is protected by the creation of an -OTBS group (down left). After which the oxazolidinone is removes to form a terminal alcohol (far right). | + | [2]In the following two steps the chiral alcohol is protected by the creation of an -OTBS group (down left). After which the oxazolidinone is removes to form a terminal alcohol (far right). |
| + | |||
| + | [3] | ||
Revision as of 15:11, 15 May 2010
Sherburn's Total Synthesis of (-)-Arctigenin
The following synthesis begins at the top left and follows the respective arrows to the bottom left product, (-)-Arctigenin.
[1]The first step involves the formation of the boron enolate of the oxazolidinone (right structure), which then is reacted with the given aldehyde(left) to produce this first structure in an approximate 92% yield.
[2]In the following two steps the chiral alcohol is protected by the creation of an -OTBS group (down left). After which the oxazolidinone is removes to form a terminal alcohol (far right).
[3]
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 12:55, 19 May 2010 | 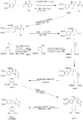 | 2,145 × 3,108 (131 KB) | Terpstra (talk | contribs) | |
| 14:31, 15 May 2010 |  | 504 × 799 (25 KB) | Terpstra (talk | contribs) | Sherburn's Total SYnthesis of (-)-Arctigenin |
- You cannot overwrite this file.
File usage
The following page links to this file: