Difference between revisions of "File:Sherburn's Total Synthesis of (-)-Artigenin.png"
(Steps of synthesis unfinnished) |
(Edited numbering system (InChI need work TMS vs TBS)) |
||
| Line 4: | Line 4: | ||
| − | + | (I)The first step involves the formation of the boron enolate of the oxazolidinone [1], which then is reacted with the given aldehyde[2] to produce this first structure [3] in an approximate 92% yield. | |
| − | + | (II)In the following two steps the chiral alcohol is protected by the creation of an -OTBS group [4]. After which the oxazolidinone is removed to form a terminal alcohol [5]. | |
| − | [ | + | (III)Separately the aldehyde [6], is reduced to an alcohol [7], the alcohol is reacted with thiophosgene (CSCl2) to produce a very reactive C-Cl bond in place of the alcohol[8]. |
| − | + | (IV)The terminal alcohol from structure [5] reacts with the C-Cl bond from structure [8] to produce a combined product [9] | |
| − | + | (V)(add link to radical rxn here) | |
| + | |||
| + | (VI) | ||
| + | |||
| + | (VII) | ||
| + | |||
| + | (VIII) | ||
| + | |||
| + | |||
| + | |||
| + | == InChI == | ||
| + | |||
| + | [1]InChI=1/C9H10O3/c1-11-8-4-3-7(6-10)5-9(8)12-2/h3-6H,1-2H3 | ||
| + | |||
| + | [2]InChI=1/C14H15NO3/c1-2-6-13(16)15-12(10-18-14(15)17)9-11-7-4-3-5-8-11/h2-8,12H,9-10H2,1H3/b6-2+/t12-/m0/s1 | ||
| + | |||
| + | [3]InChI=1/C23H25NO6/c1-4-18(21(25)16-10-11-19(28-2)20(13-16)29-3)22(26)24-17(14-30-23(24)27)12-15-8-6-5-7-9-15/h4-11,13,17-18,21,25H,1,12,14H2,2-3H3/t17-,18-,21+/m0/s1 | ||
| + | |||
| + | [4]InChI=1/C26H33NO5Si/c1-7-21(24(33(4,5)6)19-13-14-22(30-2)23(16-19)31-3)25(28)27-20(17-32-26(27)29)15-18-11-9-8-10-12-18/h7-14,16,20-21,24H,1,15,17H2,2-6H3/t20-,21-,24+/m0/s1 | ||
| + | |||
| + | [5]InChI=1/C16H26O3Si/c1-7-12(11-17)16(20(4,5)6)13-8-9-14(18-2)15(10-13)19-3/h7-10,12,16-17H,1,11H2,2-6H3/t12-,16+/m1/s1 | ||
[6] | [6] | ||
| Line 19: | Line 39: | ||
[8] | [8] | ||
| + | |||
| + | [9] | ||
Revision as of 00:06, 16 May 2010
Sherburn's Total Synthesis of (-)-Arctigenin
The following synthesis begins at the top left and follows the respective arrows to the bottom left product, (-)-Arctigenin.
(I)The first step involves the formation of the boron enolate of the oxazolidinone [1], which then is reacted with the given aldehyde[2] to produce this first structure [3] in an approximate 92% yield.
(II)In the following two steps the chiral alcohol is protected by the creation of an -OTBS group [4]. After which the oxazolidinone is removed to form a terminal alcohol [5].
(III)Separately the aldehyde [6], is reduced to an alcohol [7], the alcohol is reacted with thiophosgene (CSCl2) to produce a very reactive C-Cl bond in place of the alcohol[8].
(IV)The terminal alcohol from structure [5] reacts with the C-Cl bond from structure [8] to produce a combined product [9]
(V)(add link to radical rxn here)
(VI)
(VII)
(VIII)
InChI
[1]InChI=1/C9H10O3/c1-11-8-4-3-7(6-10)5-9(8)12-2/h3-6H,1-2H3
[2]InChI=1/C14H15NO3/c1-2-6-13(16)15-12(10-18-14(15)17)9-11-7-4-3-5-8-11/h2-8,12H,9-10H2,1H3/b6-2+/t12-/m0/s1
[3]InChI=1/C23H25NO6/c1-4-18(21(25)16-10-11-19(28-2)20(13-16)29-3)22(26)24-17(14-30-23(24)27)12-15-8-6-5-7-9-15/h4-11,13,17-18,21,25H,1,12,14H2,2-3H3/t17-,18-,21+/m0/s1
[4]InChI=1/C26H33NO5Si/c1-7-21(24(33(4,5)6)19-13-14-22(30-2)23(16-19)31-3)25(28)27-20(17-32-26(27)29)15-18-11-9-8-10-12-18/h7-14,16,20-21,24H,1,15,17H2,2-6H3/t20-,21-,24+/m0/s1
[5]InChI=1/C16H26O3Si/c1-7-12(11-17)16(20(4,5)6)13-8-9-14(18-2)15(10-13)19-3/h7-10,12,16-17H,1,11H2,2-6H3/t12-,16+/m1/s1
[6]
[7]
[8]
[9]
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 12:55, 19 May 2010 | 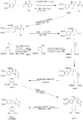 | 2,145 × 3,108 (131 KB) | Terpstra (talk | contribs) | |
| 14:31, 15 May 2010 |  | 504 × 799 (25 KB) | Terpstra (talk | contribs) | Sherburn's Total SYnthesis of (-)-Arctigenin |
- You cannot overwrite this file.
File usage
The following page links to this file: