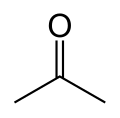
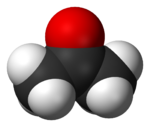
Acetone
| Acetone[1] | |
|---|---|
| IUPAC name | propanone |
| Other names | β-ketopropane, dimethyl ketone, dimethylformaldehyde, DMK, propanone, 2-propanone, propan-2-one |
| Identifiers | |
| InChI | InChI=1/C3H6O/c1-3(2)4/h1-2H3 |
| CAS number | [] |
| RTECS | AL31500000 |
| ChemSpider | |
| SMILES | |
| Properties | |
| Molecular formula | C3H6O |
| Molar mass | 58.08 g mol−1 |
| Appearance | Colorless liquid |
| Density | 0.79 g/cm3 |
| Melting point |
−94.9 °C, 178 K, -139 °F |
| Boiling point |
56.53 °C, 330 K, 134 °F |
| Solubility in water | miscible |
| Acidity (pKa) | 24.2 |
| Refractive index (nD) | 1.359 (20 °C) |
| Viscosity | 0.32 cP (20 °C) |
| Structure | |
| Molecular geometry | trigonal planar at C=O |
| Dipole moment | 2.91 D |
| Hazards | |
| EU classification | Template:Hazchem F Template:Hazchem Xi |
| R-phrases | Template:R11, Template:R36, Template:R66, Template:R67 |
| S-phrases | Template:S2, Template:S9, Template:S16, Template:S26 |
| NFPA 704 | |
| Flash point | -17 °C |
| Autoignition temp. | 465 °C |
| Explosive limits | 4.0–57.0 |
| LD50 | >2000 mg/kg, oral (rat) |
| Related compounds | |
| Other solvents | Water Ethanol Isopropanol Toluene |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Acetone is the organic compound with the formula OC(CH3)2. This colorless, mobile, flammable liquid is the simplest example of the ketones. Owing to the fact that acetone is miscible with water it serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory. More than 3 million tonnes are produced annually, mainly as a precursor to polymers.[2] Familiar household uses of acetone are as the active ingredient in nail polish remover and as paint thinner and sanitary cleaner/nail polish remover base. It is a common building block in organic chemistry. In addition to being manufactured, acetone also occurs naturally, even being biosynthesized in small amounts in the human body.
Contents
Production
Acetone is produced directly or indirectly from propene. Most commonly, in the cumene process, benzene is alkylated with propene and the resulting cumene (isopropylbenzene) is oxidized to give phenol and acetone:
- C6H5CH(CH3)2 + O2 → C6H5OH + OC(CH3)2
This conversion entails the intermediacy of cumene hydroperoxide, C6H5C(OOH)(CH3)2.
Acetone is also produced by the direct oxidation of propene with a Pd(II)/Cu(II) catalysts, akin to the Wacker process.
Older production methods
Previously, acetone was produced by the dry distillation of acetates, for example calcium acetate. During World War I acetone was produced via bacterial fermentation, as developed by Chaim Weizmann (later the first president of Israel) in order to help the British war effort.[2] This Acetone Butanol Ethanol process was abandoned due to the small yields.[2]
Biosynthesis
- See also: ketosis
Small amounts of acetone are produced in the body by the decarboxylation of ketone bodies.
Uses
About half of the world's production of acetone is consumed as a precursor to methyl methacrylate. This application begins with the initial conversion of acetone to its cyanohydrin:
- (CH3)2CO + HCN → (CH3)2C(OH)CN
In a subsequent step, the nitrile is hydrolyzed to the unsaturated amide, which is esterified:
- (CH3)2C(OH)CN + CH3OH → CH2=(CH3)CCO2CH3 + NH3
The second major use of acetone entails its condensation with phenol to give bisphenol A:
- (CH3)2CO + 2 C6H5OH → (CH3)2C(C6H4OH)2 + H2O
Bisphenol-A is a component of many polymers such as polycarbonates, polyurethanes, and epoxy resins.
Combustion
(CH3)2CO + 4O2 → 3CO2 + 3H2O
As a solvent
Acetone is a good solvent for most plastics and synthetic fibres including those used in Nalgene bottles made of polystyrene, polycarbonate and some types of polypropylene.[3]. It is ideal for thinning fiberglass resin, cleaning fiberglass tools and dissolving two-part epoxies and superglue before hardening. It is used as a volatile component of some paints and varnishes. As a heavy-duty degreaser, it is useful in the preparation of metal prior to painting; it also thins polyester resins, vinyl and adhesives.
Many millions of kilograms of acetone are consumed in the production of the solvents methyl isobutyl alcohol and methyl isobutyl ketone. These products arise via an initial aldol condensation to give diacetone alcohol.[2]
- 2 (CH3)2CO → (CH3)2C(OH)CH2C(O)CH3
Acetone is used as a solvent by the pharmaceutical industry and as a denaturation agent in denatured alcohol.[4] Acetone is also present as an excipient in some pharmaceutical products.[5]
Storage of acetylene
Although flammable itself, acetone is also used extensively as a solvent for the safe transporting and storing of acetylene, which cannot be safely pressurized as a pure compound. Vessels containing a porous material are first filled with acetone followed by acetylene, which dissolves into the acetone. One liter of acetone can dissolve around 250 liters of acetylene.[6][7]
Laboratory uses
In the laboratory, acetone is used as a polar aprotic solvent in a variety of organic reactions, such as SN2 reactions. The use of acetone solvent is also critical for the Jones oxidation. It is a common solvent for rinsing laboratory glassware because of its low cost, volatility, and ability to dissolve water. For similar reasons, acetone is also used as a drying agent. Acetone can be cooled with dry ice to -78 °C without freezing; acetone/dry ice baths are commonly used to conduct reactions at low temperatures. Acetone is fluorescent under ultraviolet light, and acetone vapor may be used as a fluorescent tracer in fluid flow experiments.[8]
Domestic and other niche uses
Acetone is often the primary component in cleaning agents such as nail polish remover. Ethyl acetate, another organic solvent, is sometimes used as well. Acetone is a component of superglue remover and it easily removes residues from glass and porcelain.
It can be used as an artistic agent; when rubbed on the back of a laser print or photocopy placed face-down on another surface and burnished firmly, the toner of the image is allowed to transfer to the destination surface.
Some automotive enthusiasts add acetone at around 1 part in 500 to their fuel, following claims of dramatic improvement in fuel economy and engine life.[9] This practice is controversial as the body of systematic testing shows that acetone has no measurable effect or may in fact reduce engine life by adversely affecting fuel system parts.[10][11] Debates on this subject and the perennial claims of a "Big Oil" cover-up intensified when the practice was addressed on the popular American TV show MythBusters in 2006, and shown to have negative effect in the televised fuel economy test.[12]
Safety
Flammability
The most common hazard associated with acetone is its extreme flammability. It auto-ignites at a temperature of 465 °C (869 °F). At temperatures greater than acetone's flash point of −20 °C (−4 °F), air mixtures of between 2.5% and 12.8% acetone, by volume, may explode or cause a flash fire. Vapors can flow along surfaces to distant ignition sources and flash back. Static discharge may also ignite acetone vapors.[13]
Acetone peroxide
When oxidized, acetone forms acetone peroxide as a byproduct, which is a highly unstable compound. It may be formed accidentally, e.g. when waste hydrogen peroxide is poured into waste solvent containing acetone. Acetone peroxide is more than ten times as friction and shock sensitive as nitroglycerin. Due to its instability, it is rarely used, despite its easy chemical synthesis.
Toxicology
Acetone is believed to exhibit only slight toxicity in normal use, and there is no strong evidence of chronic health effects if basic precautions are followed.[14]
At very high vapor concentrations, acetone is irritating and, like many other solvents, may depress the central nervous system. It is also a severe irritant on contact with eyes, and a potential pulmonary aspiration risk. In one documented case, ingestion of a substantial amount of acetone led to systemic toxicity, although the patient eventually fully recovered.[15] Some sources estimate LD50 for human ingestion at 1.159 g/kg; LD50 inhalation by mice is given as 44 g per cubic meter, over 4 hours.[16]
Interestingly, acetone has been shown to have anticonvulsant effects in animal models of epilepsy, in the absence of toxicity, when administered in millimolar concentrations.[17] It has been hypothesized that the high-fat low-carbohydrate ketogenic diet used clinically to control drug-resistant epilepsy in children works by elevating acetone in the brain.[17]
Environmental effects
Acetone evaporates rapidly, even from water and soil. Once in the atmosphere, it is degraded by UV light with a 22-day half-life. Acetone dissipates slowly in soil, animals, or waterways since it is sometimes consumed by microorganisms;[18] however, it is a significant issue with respect to groundwater contamination due to its high solubility in water. The LD50 of acetone for fish is 8.3 g/l of water (or about 0.8%) over 96 hours, and its environmental half-life is about 1 to 10 days. Acetone may pose a significant risk of oxygen depletion in aquatic systems due to the microbial activity consuming it.[19]
References
- ↑ Merck Index, 11th Edition, 58.
- ↑ 2.0 2.1 2.2 2.3 Stylianos Sifniades, Alan B. Levy, “Acetone” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- ↑ NALGENE Labware - Technical Data
- ↑ Weiner, Myra L.; Lois A. Kotkoskie (1999). Excipient Toxicity and Safety, 32. ISBN 0824782100, 9780824782108.
- ↑ http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm
- ↑ Mine Safety and Health Administration (MSHA) - Safety Hazard Information - Special Hazards of Acetylene
- ↑ History - Acetylene dissolved in acetone
- ↑ A. Lozano, B. Yip and R. K. Hanson (1992). "Acetone: a tracer for concentration measurements in gaseous flows by planar laser-induced fluorescence". Exp. Fluids 13: 369–376. doi:10.1007/BF00223244.
- ↑ Louis LaPonte (2007-02-13). Acetone in Fuels (A Study of Dimethylketone or Propanone). Smartgas.net. Retrieved on 2007-06-06.
- ↑ Tom and Ray Magliozzi (2006-01-21). Click and Clack Talk Cars. Independent Record. Retrieved on 2007-06-06.
- ↑ Can adding Acetone to fuel increase mpg by 15 to 35%?. Snopes.com Message Board. Retrieved on 2007-06-06.
- ↑ MythBusters (Season 4, Episode 53)
- ↑ Acetone MSDS
- ↑ http://ccohs.ca/oshanswers/chemicals/chem_profiles/acetone/basic_ace.html
- ↑ Canadian Centre for Occupational Health and Safety. Health Effects of Acetone. Retrieved on 2008-10-21.
- ↑ Safety (MSDS) data for propanone
- ↑ 17.0 17.1 Likhodii SS, Serbanescu I, Cortez MA, Murphy P, Snead OC 3rd, Burnham WM (2003). "Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet". Ann Neurol. 54 (2): 219–226. doi:10.1002/ana.10634.
- ↑ tf21
- ↑ http://jmloveridge.com/cosh/Acetone.pdf
External links
- International Chemical Safety Card 0087
- National Pollutant Inventory: Acetone
- NIOSH Pocket Guide to Chemical Hazards
- Calculation of vapor pressure, liquid density, dynamic liquid viscosity, surface tension of acetone
- Hazardous substances databank entry at the national library of medicine
- Fisher Chemical MSDS
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Acetone". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |