Tert-Butyl chloride
| Tert-Butyl chloride | |
|---|---|
| IUPAC name | 2-chloro-2-methylpropane |
| Other names | 1,1-dimethylethyl chloride 1-chloro-1,1-dimethylethane 2-chloroisobutane 2-methyl-2-chloropropane chlorotrimethylmethane trimethylchloromethane t-butyl chloride tert-butyl chloride t-BuCl UN 1127 |
| Identifiers | |
| InChI | InChI=1/C4H9Cl/c1-4(2,3)5/h1-3H3 |
| CAS number | [] |
| EC number | |
| RTECS | TX5040000 |
| PubChem | |
| SMILES | |
| Properties | |
| Chemical formula | C4H9Cl |
| Molar mass | 92.57 g/mol |
| Appearance | Colorless liquid |
| Density | 0.84 g cm−3 |
| Melting point |
−26 °C |
| Boiling point |
51 °C |
| Solubility in water | Sparingly sol in water, miscible with alcohol and ether |
| Vapor pressure | 34.9 kPa (20 °C) |
| Hazards | |
| EU classification | Flammable (F) |
| R-phrases | Template:R12, Template:R36/37/38 |
| S-phrases | Template:S7, Template:S9, Template:S16, Template:S29, Template:S33 |
| NFPA 704 | |
| Flash point | −9 °C (open cup) −23 °C (closed cup) |
| Autoignition temp. | 540 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
tert-Butyl chloride is a colorless, liquid organic compound at room temperature. It is sparingly soluble in water, with a tendency to undergo spontaneous solvolysis when dissolved into it. The compound is flammable and volatile, and its main use is as a starting molecule to carry out nucleophilic substitution reactions, to produce different substances, ranging from alcohols to alkoxide salts.
When tert-butyl chloride is dissolved in water, a polar and protic solvent, the bulky chloride substituent is carried away by it, and isolated from the aliphatic chain, causing an heterolitic rupture of the compound, giving rise to a carbocation which eventually becomes a tertiary alcohol after a water molecule reacts with it, releasing hydrochloric acid as the final product. If a different, stronger nucleophilic agent is present at the moment of reaction, reaction product may not be an alcohol, but a tertiary carbon with the nucleophile as a substituent.
Synthesis
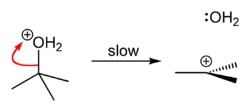
tert-Butyl chloride can be synthesized in the laboratory by the SN1 reaction of tert-Butanol with concentrated hydrochloric acid, as shown below.
 |
 |
 |
The overall reaction, therefore, is:
Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the Step 2 allows the SN1 mechanism to be followed, whereas a primary alcohol would follow an SN2 mechanism.
See also
External links
- Safety MSDS data
- Preparation 2-chloro-2-methylpropane
- http://www.cerlabs.com/experiments/10875407331.pdf
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Tert-Butyl chloride". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |