Research:Walker group/transacylation
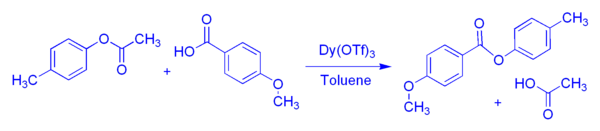
The transacylation of esters involves the reaction of an ester (typically an acetate ester) with a carboxylic acid to produce a new ester, along with a new carboxylic acid (typically acetic acid). We discovered that this process can occur in high yield with acetate esters, in the presence of hydrated dysprosium(III) triflate, as long as the process is driven by removal of the product acid (acetic acid). For example, p-tolyl acetate reacts with p-anisic acid to produce a new ester, p-tolyl p-anisate, and acetic acid: