Nakada's synthesis of digitoxigenin
Digitoxin and digoxin make up the drug digitalis, which is used as a cardiac drug to treat cardiac congestion and cardiac arrythmias. The mechanism within the heart involves increasing intracellular Na+ and Ca2+ while decreasing intracellular K+. The increased Ca2+ promotes muscle contraction and cardiac contractile force. Digitalis has also been reported to exhibit anti-cancer activity.
A glycoside consists of part sugar and part nonsugar. The sugar portion is called the glycone and the nonsugar portion is called the aglycone, or genin. Digitoxigenin is the aglycone of digitoxin. The R group of digitoxigenin is what defines the class of cardiac drug.
Cardiac drug structure
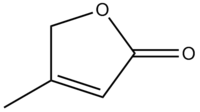
The R group of digitoxigenin is shown below:
Retrosynthetic Analysis
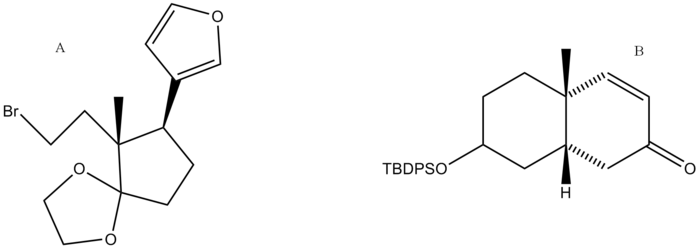
The synthesis of digitoxigenin was developed by Honma and Nakada. A retrosynthetic analysis was performed and the final product was broken down into two major retrosynthetic pieces, shown below (pieces A and B).
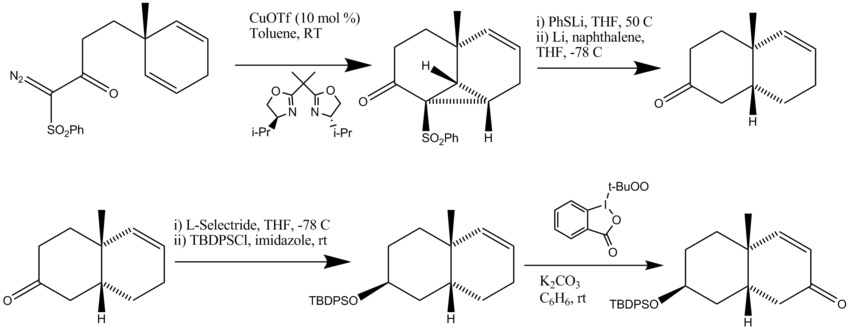
Synthesis of Part B
- The first step was a catalytic asymmetric intramolecular cyclopropanation
- The cyclopropane ring was opened and the phenylthio group/phenylsulfonyl group was removed.
- The starting material was first reduced with L-selectride to form the correct stereoselective isomer. The ketone was then protected using a TBDPS protecting group.
- Allylic oxidation was performed using a reagent reported by Ochiai to form retrosynthetic piece B