Nakada's synthesis of digitoxigenin
Digitoxin and digoxin make up the drug digitalis, which is used as a cardiac drug to treat cardiac congestion and cardiac arrythmias. The mechanism within the heart involves increasing intracellular Na+ and Ca2+ while decreasing intracellular K+. The increased Ca2+ promotes muscle contraction and cardiac contractile force. Digitalis has also been reported to exhibit anti-cancer activity.
Contents
Cardiac drug structure
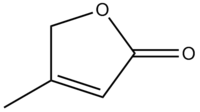
A glycoside consists of part sugar and part nonsugar. The sugar portion is called the glycone and the nonsugar portion is called the aglycone, or genin. Digitoxigenin is the aglycone of digitoxin. The R group of digitoxigenin is what defines the class of cardiac drug.
The R group of digitoxigenin is shown below:
Retrosynthetic Analysis
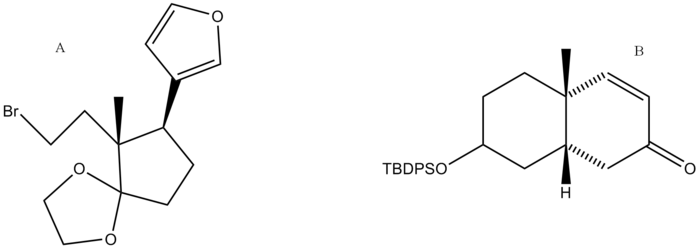
The synthesis of digitoxigenin was developed by Honma and Nakada. A retrosynthetic analysis was performed and the final product was broken down into two major retrosynthetic pieces, shown below (pieces A and B).
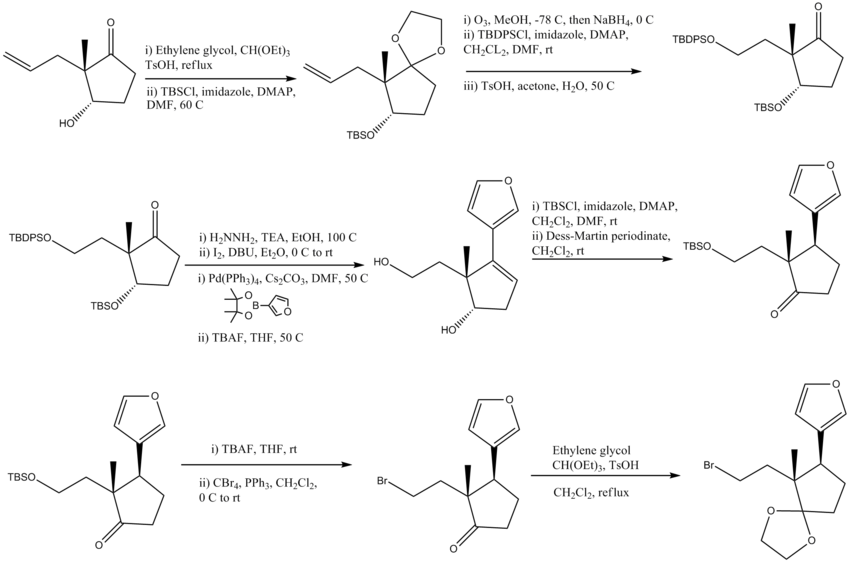
Synthesis of Part A
- Ethylene ketal formation (protecting group), addition of protecting group TBSO
- Conversion of an alkene to an alcohol and protection of the alcohol with TBDPSO, Ketal returned to ketone form with base
- Wolff-Kishner Reduction, Conversion to -I, Suzuki coupling reaction, Removal of protecting groups TBDPSO and TBSO
- Protection of the primary alcohol with a TBS protecting group, Oxidation of the secondary alcohol to a ketone
- Removal of the TBS protecting group, Conversion of the alcohol to a bromine
- Formation of a ketal from the ketone and final step of product A
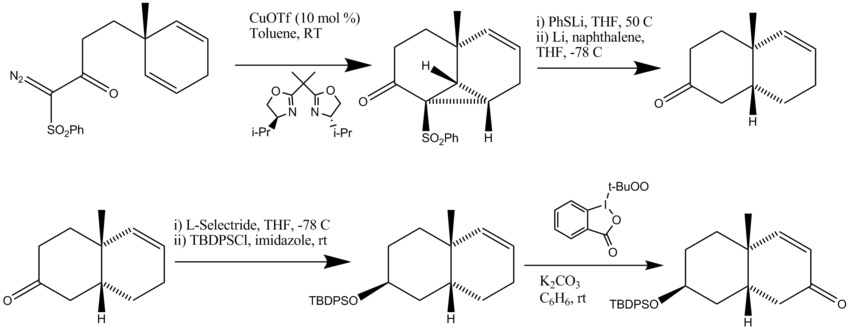
Synthesis of Part B
- The first step was a catalytic asymmetric intramolecular cyclopropanation
- The cyclopropane ring was opened and the phenylthio group/phenylsulfonyl group was removed.
- The starting material was first reduced with L-selectride to form the correct stereoselective isomer. The ketone was then protected using a TBDPS protecting group.
- Allylic oxidation was performed using a reagent reported by Ochiai to form retrosynthetic piece B
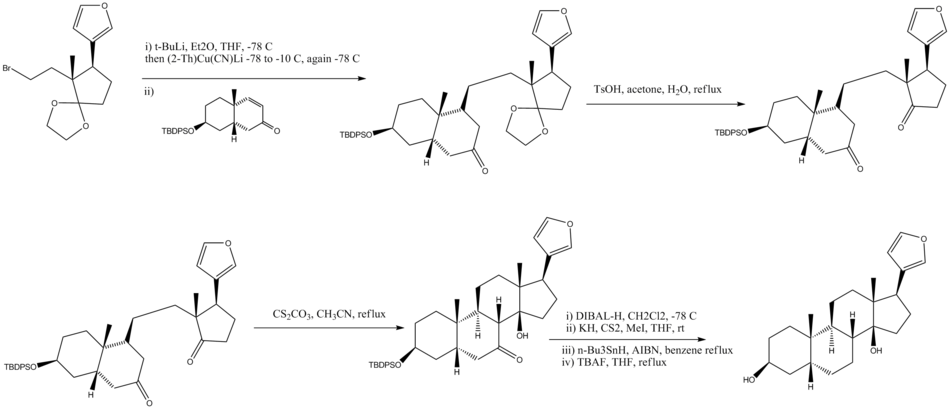
Convergent Steps
- Cuprate with lithium produces a single diastereomer
- Ethylene ketal removed under acidic conditions
- Intramolecular aldol reaction
- Deoxygenation in four steps, TBDPS protecting group removed
References
Honma, Masahiro and Masahisa Nakada. Tet Letters 48(2007) 1541-1544. Desai, Umesh. www.people.vcu.edu/~urdesai/car.htm, accessed 16 April 2008. Haux, J. Medical Hypotheses (1999) 53(6), 543-54.