Caesium chloride
| Caesium chloride | |
|---|---|

| |

| |
| Other names | Cesium chloride |
| Identifiers | |
| CAS number | [] |
| EC number | |
| Properties | |
| Chemical formula | CsCl |
| Molar mass | 168.36 g/mol |
| Appearance | white solid hygroscopic |
| Density | 3.99 g/cm3 |
| Melting point |
645 °C |
| Boiling point |
1297 °C (vaporizes) |
| Solubility in water | 162 g/100 mL (1 °C) |
| Solubility | soluble in ethanol [1] |
| Structure | |
| Crystal structure | Caesium chloride (see text) |
| Coordination geometry | simple cubic |
| Related compounds | |
| Other anions | Caesium fluoride Caesium bromide Caesium iodide |
| Other cations | Lithium chloride Sodium chloride Potassium chloride Rubidium chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Caesium chloride is the chemical compound with the formula CsCl. This colorless solid is an important source of caesium ions in a variety of applications. CsCl is also well known as a structural type.
Preparation
Caesium chloride can be prepared by the reaction of caesium hydroxide or caesium carbonate with hydrochloric acid: the resulting salt is purified by recrystallization.
Crystal structure
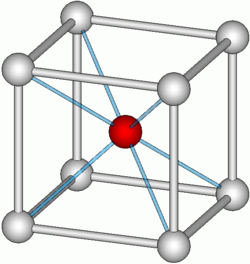
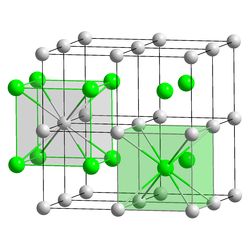
The caesium chloride structure is composed of a primitive cubic lattice with a two atom basis, where both atoms have eight-fold coordination. The chloride atoms lie upon the lattice points at the edges of the cube, while the caesium atoms lie in the holes in the center of the cubes. This structure is shared with CsBr and CsI and many intermetallic compounds. In contrast, the other alkaline halides have the sodium chloride structure.[2] When both ions are similar in size (Cs+ ionic radius 174 pm for this coordination number, Cl− 181 pm) the CsCl structure is adopted, when they are different (Na+ ionic radius 102pm, Cl− 181 pm) the sodium chloride structure is adopted.
 |
 |
unit cell of the CsCl structure |
coordination of Cs and Cl in CsCl |
Uses
Caesium chloride is also widely used in the centrifugation in a technique known as isopycnic centrifugation. Centrifugal and diffusive forces establish a density gradient which allows separation of mixtures on the basis of their molecular density. This technique allows separation of DNA of different densities (e.g. DNA fragments with differing A-T or G-C content).[3]
Radioisotopes of caesium chloride are used in nuclear medicine, including treatment of cancer. In the production of radioactive sources, it is normal to choose a chemical form of the radioisotope which would not be readily dispersed in the environment in the event of an accident. For instance, radiothermal generators (RTGs) often use strontium titanate, which is insoluble in water. For teletherapy sources, however, the radioactive density (Ci in a given volume) needs to be very high, which is not possible with known insoluble caesium compounds. A thimble-shaped container of radioactive caesium chloride provides the active source. In the Goiânia accident, such a source was reptured, leading to several deaths.
Miscellaneous applications
Caesium chloride (non-radioactive) is also promoted as an alternative cancer therapy.[4] Caesium chloride is used in the preparation of electrically conducting glasses.[5]
References
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ↑ Manfred Bick and Horst Prinz "Cesium and Cesium Compounds” in Ullmann’s Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim.DOI:10.1002/14356007.a06_153.
- ↑ Sartori H. E. "Cesium therapy in cancer patients." Pharmacol Biochem Behav. 1984;21 Suppl 1:11-3.PMID: 6522427.
- ↑ Tver'yanovich, Y. S. et al. (1998). Glass Phys. Chem., 24, 446.
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Caesium chloride". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |