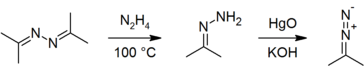Azine
Azines are a functional class of organic compounds, formed from the condensation reaction of two equivalents of an aldehyde or ketone with one equivalent of hydrazine.[1][2] They may be further classified as aldazines or ketazines, depending on the nature of the carbonyl compound.[3][4]
Preparation
Azines may be prepared by the direct reaction of a carbonyl compound with hydrazine hydrate:[5] the reaction is exothermic.[6]
Reactions and uses
Azines have been used as precursors to hydrazones[5][7] and diazo compounds.[7][8][9]
Azines have been used as sources of hydrazine produced in situ, for example in the production of herbicide precursor 1,2,4-triazole.[10]
Nomenclature
Azines may be named by substitutive or functional class nomenclature.[11][12] In functional class nomenclature, the functional modifier "azine" is appended to the name of the carbonyl compound: hence, "acetone azine". In substitutive nomenclature, azines are named as derivatives of hydrazine: hence, "diisopropylidenehydrazine". In the presence of groups of higher seniority, the prefixes "hydrazinylidene" and "hydrazinediylidene" are used.[13]
Unsymmetrical azines, that is compounds of the type X=N–N=Y with X ≠ Y, are not named as azines: in the absence of other functional groups having higher seniority, they can be named as substituted hydrazones.[14]
References
- ↑ Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67 (8-9), 1307–75 at 1321. DOI: 10.1351/pac199567081307.
- ↑ azines, <http://goldbook.iupac.org/A00557.html> (accessed 2 July 2010), Compendium of Chemical Terminology Internet edition; International Union of Pure and Applied Chemistry (IUPAC).
- ↑ Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67 (8-9), 1307–75 at 1312, 1348. DOI: 10.1351/pac199567081307.
- ↑ aldazines, <http://goldbook.iupac.org/A00207.html> (accessed 2 July 2010), Compendium of Chemical Terminology Internet edition; International Union of Pure and Applied Chemistry (IUPAC). ketazines, <http://goldbook.iupac.org/K03377.html> (accessed 2 July 2010), Compendium of Chemical Terminology Internet edition; International Union of Pure and Applied Chemistry (IUPAC).
- ↑ 5.0 5.1 Day, A. C.; Whiting, M. C. Acetone hydrazone. Org. Synth. 1970, 50, 3, <http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0010>; Coll. Vol., 6, 10.
- ↑ Gilbert, E. C. Studies on Hydrazine. The Hydrolysis of Dimethylketazine and the Equilibrium between Hydrazine and Acetone. J. Am. Chem. Soc. 1929, 51 (11), 3394–3409. DOI: 10.1021/ja01386a032.
- ↑ 7.0 7.1 Staudinger, H.; Gaule, Alice Vergleich der Stickstoff-Abspaltung bei verschiedenen aliphatischen Diazoverbindungen. Ber. Dtsch. Chem. Ges. 1916, 49 (2), 1897–1918. DOI: 10.1002/cber.19160490245.
- ↑ Day, A. C.; Raymond, P.; Southam, R. M.; Whiting, M. C. The preparation of secondary aliphatic diazo-compounds from hydrazones. J. Chem. Soc. C 1966, 467–69. DOI: 10.1039/J39660000467.
- ↑ Andrews, S. D.; Day, A. C.; Raymond, P.; Whiting, M. C. 2-Diazopropane. Org. Synth. 1970, 50, 27, <http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0392>; Coll. Vol., 6, 392.
- ↑ Nagata, Nobuhiro; Nishizawa, Chiharu; Kurai, Toshikiyo (Mitsubishi Gas Chemical Co.) Method of producing 1,2,4-triazole. US Patent 6002015, issued 14 December 1999.
- ↑ Rule R-5.6.6.3. In A Guide to IUPAC Nomenclature of Organic Compounds; IUPAC Recommendations 1993; Blackwell Science: Oxford, 1993; p 105. ISBN 0-632-03488-2, <http://www.acdlabs.com/iupac/nomenclature/93/r93_469.htm>.
- ↑ Draft Rule P-68.3.1.2.3. In Nomenclature of Organic Chemistry; IUPAC Provisional Recommendations 2004; IUPAC, 2004, <http://old.iupac.org/reports/provisional/abstract04/favre_310305.html>.
- ↑ Draft Rule P-53.6.2. In Nomenclature of Organic Chemistry; IUPAC Provisional Recommendations 2004; IUPAC, 2004, <http://old.iupac.org/reports/provisional/abstract04/favre_310305.html>.
- ↑ Rule C-923.2. In Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H; IUPAC Recommendations 1979; Pergamon: Oxford, 1979. ISBN 0-08022-369-9, <http://www.acdlabs.com/iupac/nomenclature/79/r79_623.htm>.
| Error creating thumbnail: Unable to save thumbnail to destination |
This page is currently licensed under the Creative Commons Attribution 3.0 Unported license and any later versions of that license. |