Lebreton's synthesis of (-)-lobeline
Contents
Background and Introduction
- ( – )-Lobelia is one of the many piperidine alkoloids found in lobelia inflata, also known as asthma plant. The most important part of the plant is the seed as it contains the most lobeline. Listed are some of very known uses of the herb for centuries; treatment of asthma, respiratory stimulant, insect repellent, drug deterrent, tobacco; which inherits another common name to the herb: Indian tobacco, entheogen, ointment but most importantly for its effects on central nervous system. Although shows no structural resemblance to nicotine, since from late 1930’s, its effect on the nicotine receptor have been realized and even used in drugs for treatment of smoking cessation. Due to its highly diverse effects, it is still considered one of the most medically important plants and is in the center of intense research for possible drug synthesis. Here in the poster, we provided the stereoselective synthesis of the ( – )-Lobelia, using benzaldehyde as a starting molecule.
Origin and Features
- Lobelia Inflata is native to North America, from southeastern Canada through the eastern United States to Alabama and west to Kansas. It is an erect annual or biennial herb and usually grows upto 1-2 ft high. It belongs to Campanulaceae family and Lobelioideae subfamily. The flowers are pale violet in color and tinted yellow on the inside. The branched leaves are sessile and toothed, usually being 8 cm long. They vary from ovate or oblong below to foliatious above. The stem is usually covered in tiny hairs and the root is slender with yellowish-white color. The plant develops two-celled capsuled fruits which contain brownish-colored seeds with ( – )-Lobelia in them.
Mode of Action
- Studies with lobeline like structures have shown that full structure is necessary for the effective functioning of the molecule. It is believed that, it acts on acetycholine receptors which can be divided into two major subgroups; muscarinic and nicotinic. nAChRs are known for their involvement in cognitive, motor and behavioral systems. It is the hydrogen bonding that covalently attaches the lobeline to the receptors. However since the nicotine and lobeline show differential effects in behavioral and neurochemical studies suggest that these drugs may not be acting via common mechanism even though lobeline has been considered to be a nicotinic agonist. One novel mechanism proposed that just like nicotine, lobeline evokes the dopamine release. However lobeline induced dopamine release was calcium-independent and was insensitive to mecamylamine, a non-competitive nicotinic receptor antagonist that blocks the ion channel. In addition, at weak concentrations lobeline blocked nicotine evoked dopamine release indicating its dual agonist and antagonist function. The inhibition of dopamine uptake into synaptic vesicles occurs via VMAT2, which results in a corresponding redistribution of presynaptic dopamine storage and an increase in the cytosolic dopamine pool. The resulting increase in the cytosolic dopamine leads to an increase in dihydroxyphenylacetic acid, DOPAC, as a result of metabolism of the cytosolic dopamine pooled by monoamine oxidase. This redistribution ultimately leads to a decrease in the cytosolic dopamine pool available for the reverse transport by the dopamine transporter. So by acting as an indirect antagonist of DA receptors, lobeline antagonises the effect of psychostimulatnts which produce their effects by activation of the dopaminergicc system which suggest that lobeline and its analogues could be novel class of therapeutic agents with a great potential for the treatment of psychostimulant abuse.
Synthesis
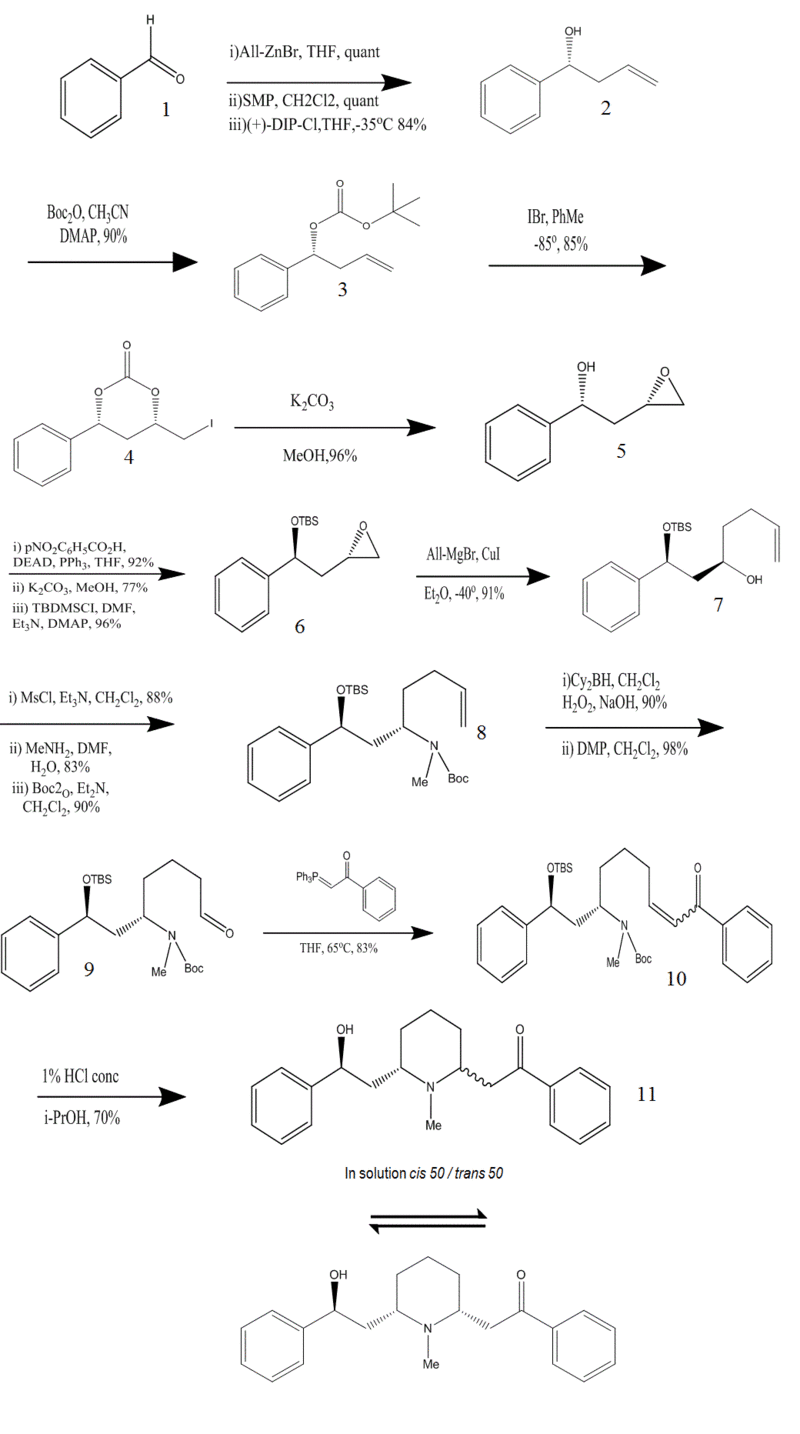
Following are the InChI codes for the numbered compounds in the synthesis
1) InChI=1/C7H6O/c8-6-7-4-2-1-3-5-7/h1-6H/i1-12,2-12,3-12,4-12,5-12,6-12,7-12,8-16
2) InChI=1/C10H12O/c1-2-6-10(11)9-7-4-3-5-8-9/h2-5,7-8,10-11H,1,6H2/t10-/m1/s1/i1-12,2-12,3-12,4-12,5-12,6-12,7-12,8-12,9-12,10-12,11-16
3) InChI=1/C15H20O3/c1-5-9-13(12-10-7-6-8-11-12)17-14(16)18-15(2,3)4/h5-8,10-11,13H,1,9H2,2-4H3/t13-/m1/s1/i1-12,2-12,3-12,4-12,5-12,6-12,7-12,8-12,9-12,10-12,11-12,12- 12,13-12,14-12,15-12,16-16,17-16,18-16
4) InChI=1/C11H11IO3/c12-7-9-6-10(15-11(13)14-9)8-4-2-1-3-5-8/h1-5,9-10H,6-7H2/t9-,10+/m0/s1/i1-12,2-12,3-12,4-12,5-12,6-12,7-12,8-12,9-12,10-12,11-12,12+18,13-16,14-16,15-16
5) InChI=1/C10H12O2/c11-10(6-9-7-12-9)8-4-2-1-3-5-8/h1-5,9-11H,6-7H2/t9-,10+/m0/s1/i1-12,2-12,3-12,4-12,5-12,6-12,7-12,8-12,9-12,10-12,11-16,12-16
6) InChI=1/C16H26O2Si/c1-16(2,3)19(4,5)18-15(11-14-12-17-14)13-9-7-6-8-10-13/h6-10,14-15H,11-12H2,1-5H3/t14-,15?/m0/s1/i1-12,2-12,3-12,4-12,5-12,6-12,7-12,8-12,9-12,10-12,11-12,12-12,13-12,14-12,15-12,16-12,17-16,18-16,19-28
7) InChI=1/C19H32O2Si/c1-7-8-14-17(20)15-18(16-12-10-9-11-13-16)21-22(5,6)19(2,3)4/h7,9-13,17-18,20H,1,8,14-15H2,2-6H3/t17-,18?/m1/s1/i1-12,2-12,3-12,4-12,5-12,6-12,7- 12,8-12,9-12,10-12,11-12,12-12,13-12,14-12,15-12,16-12,17-12,18-12,19-12,20-16,21-16,22-28
8) InChI=1/C25H43NO3Si/c1-11-12-18-21(26(8)23(27)28-24(2,3)4)19-22(20-16-14-13-15-17-20)29-30(9,10)25(5,6)7/h11,13-17,21-22H,1,12,18-19H2,2-10H3/t21-,22?/m0/s1/i1-12,2-12,3-12,4-12,5-12,6-12,7-12,8-12,9-12,10-12,11-12,12-12,13-12,14-12,15-12,16-12,17-12,18-12,19-12,20-12,21-12,22-12,23-12,24-12,25-12,26-14,27-16,28-16,29-16,30-28
9) InChI=1/C25H43NO4Si/c1-24(2,3)29-23(28)26(7)21(17-13-14-18-27)19-22(20-15-11-10-12-16-20)30-31(8,9)25(4,5)6/h10-12,15-16,18,21-22H,13-14,17,19H2,1-9H3/t21-,22?/m0/s1/i1-12,2-12,3-12,4-12,5-12,6-12,7-12,8-12,9-12,10-12,11-12,12-12,13-12,14-12,15-12,16-12,17-12,18-12,19-12,20-12,21-12,22-12,23-12,24-12,25-12,26-14,27-16,28-16,29-16,30-16,31-28
10) InChI=1/C27H35NO4/c1-27(2,3)32-26(31)28(4)23(20-25(30)22-16-10-6-11-17-22)18-12-7-13-19-24(29)21-14-8-5-9-15-21/h5-6,8-11,13-17,19,23,25,30H,7,12,18,20H2,1-4H3/b19-13-/t23-,25-/m0/s1/i1-12,2-12,3-12,4-12,5-12,6-12,7-12,8-12,9-12,10-12,11-12,12-12,13-12,14-12,15-12,16-12,17-12,18-12,19-12,20-12,21-12,22-12,23-12,24-12,25-12,26-12,27-12,28-14,29-16,30-16,31-16,32-16
11) InChI=1/C28H41NO2Si/c1-28(2,3)32(5,6)31-27(23-16-11-8-12-17-23)21-25-19-13-18-24(29(25)4)20-26(30)22-14-9-7-10-15-22/h7-12,14-17,24-25,27H,13,18-21H2,1-6H3/t24-,25+,27?/m1/s1/i1-12,2-12,3-12,4-12,5-12,6-12,7-12,8-12,9-12,10-12,11-12,12-12,13-12,14-12,15-12,16-12,17-12,18-12,19-12,20-12,21-12,22-12,23-12,24-12,25-12,26-12,27-12,28-12,29-14,30-16,31-16,32-28
Key Steps in Synthesis
- One of the most important key steps in the mechanism was the synthesis of the chiral homoallylic alcohol. Once the desired enatioselectivity of the alcohol was achieved, the chirality induction would direct the diastereoslective epoxidation of double bond to afford the syn epoxy alcohol and the inversion of the absolute configuration of the alcohol would lead to the desired anti-epoxy alcohol. Then, regioselective ring opening of the terminal alcohol would be carried out with allyorganometallic reagent. This reaction in the synthesis would not only lead to the desired carbon skeleton but also introduce a terminal double bond precursor of primary alcohol and liberate the secondary alcohol. To do this effectively, a three step sequence reaction, based on an iodocyclization procedure was carried out. First tert-butyl dicarbonate was prepared then; distereoslective iodine-induced electrophilic cyclization was performed by treatment with IBr at low temperature. Finally basic condition was provided which led to the syn-epoxy alcohol. Protecting groups were chosen such that they were prone to cleavage in acidic conditions. Final step was the conversion of aldehyde to unsaturated aryl ketone via Wittig olefination. It is worth to mention that although in crystalline form, it was all cis isomer obtained, in solution both isomers were present in 1:1 ratio due to retro-Michael reaction.
Medical History
- When first discovered, Lobeline had been used in the treatment of respiratory problems especially asthma and bronchitis and its action was explained through the activation of the carotid and aortic body chemoreceptors at therapeutic doses. It was shown that lobeline relaxes the tissues and favors expectoration when a large quantity of mucus is secreted. Due its effective respiratory tract clearing feature, it also helped in the treatment of victims who have been electrocuted or asphyxiated by toxic gases. Another major use of lobeline was as a smoking deterrent since it is believed to be a substitute of nicotine. Until 1993, when it was prohibited by FDA due to its inefficiency, it was widely used in many drugs some being Nicoban, CigArest and NicFit. The recent studies show that its inefficiency was actually due to its bioavailabilty. Its role in the treatment of CNS diseases and pathologies is another main feature of this chemical. It is shown in rodents that it greatly enhances memory due to its involvement in cholinergic mechanisms of neurotransmission. It also stimulates the vomiting center in the CNS and was shown to produce sympathetic and parasympathetic effects through the stimulation of the 34salivation and diarrhea.
Poster
-Here is the link to the poster we presented on 21st of April. File:Banu Poster041610.ppt
References
- 1. Compere, D., Marazano, C., & Das, B. C. (1999). Enantioselective Access to Lobelia Alkoloids. Journal of Organic Chemistry , 4528-4532.
- 2. Damaj, M. I., Patrick, G. S., Creasy, K. R., & Martin, B. R. (1997). Pharmacology of Lobeline, A Nicotinic Receptor Ligand. The Journal of Pharmacology and Experimental Therapeutics , 410-419.
- 3. Dwoskin, L. P., & Crooks, P. A. (2002). A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochemical Pharmacology , 89-98.
- 4. Felpin, F.-X., & J, L. (2004). History, chemistry and biology of alkoloids from Lobelia inflata. Tetrahedron , 10127-10153.
- 5. Felpin, F.-X., & Lebreton, J. (2002). A Highly Selective Asymmetric Synthesis of ( – )-Lobeline and ( – )-Sedamine . Journal of Organic Chemistry , 9192-9199.