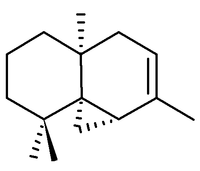
Thujopsene
Thujopsene
| Thujopsene | |
|---|---|
| |
| IUPAC name | (1aS,4aS,8aS)-2,4a,8,8-tetramethyl-1,1a,4,4a,5,6,7,8-octahydrocyclopropa[d]naphthalene |
| Other names | Widdrene |
| Identifiers | |
| InChI | InChI=1/C15H24/c1-11-6-9-14(4)8-5-7-13(2,3)15(14)10-12(11)15/h6,12H,5,7-10H2,1-4H3/t12-,14-,15-/m0/s1 |
| InChIKey | WXQGPFZDVCRBME-QEJZJMRPBP |
| CAS number | [] |
| ChemSpider | |
| PubChem | |
| SMILES | |
| Properties | |
| Chemical formula | C15H24 |
| Molar mass | 204.35 g/mol |
| Appearance | Clear, colourless liquid |
| Density | 0.936 g/mL (liquid at 20°C) |
| Boiling point |
258-260 °C, 271 K, -178 °F |
| Solubility in water | Very low |
| Specific rotation [α]D | −110° (CHCl3) |
| Hazards | |
| Main hazards | flammable |
| NFPA 704 | |
| Flash point | 104 °C |
| Related compounds | |
| Other alkene | Cedrene, Germacrene |
| Other compounds | Cedrol, Widdrol |
| Template:Tick(what is this?) (verify) Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Thujopsene is a tricyclic sesquiterpene commonly found in oils isolated from trees of the Cupressaceae (cypress) family, especially the geni Thujopsis, Juniperus (juniper) and Widdringtonia. Thujopsene has a sweet smell of pencil wood[1], and it undergoes acetylation with rearrangement to produce a compound with a woody odour which is used for perfumery.
Contents
Structure
The correct structure and relative stereochemistry (cis) was first established in 1961 by T. Norin, [2] and confirmed by total synthesis in 1963.[3]. The absolute configuration was established by T. Norin in 1963,[4] confirming work on the related sesquiterpene widdrol by C. Enzell. [5]
Occurrence
Thujopsene is the principal component in several natural Cupressacea oils. It was first isolated from hiba oil by Yano (1913) and Uchida (1928)[6] . It was given the name thujopsene in 1930 by Kawamura, who assigned an incorrect structure based on a homologue of alpha-pinene.[7]
In 1958, thujopsene was identified (initially as "widdrene") in the heartwood of the five Widdringtonia species by Erdtman and Thomas. [8] Thujopsene was then identified as the principal volatile component in the essential oil from various other species, including Juniperus cedrus and Juniperus phoenicea; in 1960 Runeburg commented that "J. Phoenicea appears to be the most convenient source of thujopsene so far encountered."[9] It is also a significant component (around 30%) of the misnamed"cedar oil", isolated from Juniperus virginiana (Easter redcedar), which has been produced in very large quantities for the perfumery industry.[10]
Thujopsene content in various types of wood is given in Table 1. The total % oil indicates what percentage of the wood was isolated as an essential oil. Note that thujopsene does not occur in significant quantities in Juniperis procera[11] or Juniperus foetidissima. [12] All figures are approximate and variable.
| Species | Common name | % Thujopsene in dry wood |
% Thujopsene in distilled oil |
Ref. |
|---|---|---|---|---|
| Juniperis chinensis | Chinese juniper | 0.27 | [13] | |
| Juniperus osteosperma | Utah juniper | 0.18 | 19* | [14] |
| Juniperus virginiana | Eastern Redcedar | - | 30† | [10] |
| Juniperus thurifera | Spanish Juniper | 0.4 | - | [15] |
| Juniperus cedrus | Canary Islands Juniper | 2.2 | - | [16] |
| Juniperus phoenicea | Arâr | 2.2 | - | [9] |
| Juniperus californica | California Juniper | 0.1 | - | [11] |
* Percentage in only the neutral oil portion isolated via an acetone extraction; excludes the portion that is extractable into base.
† Percentage in the oil obtained by steam distillation.
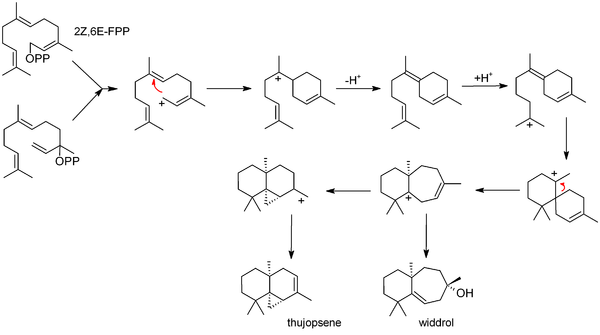
Biosynthesis
The commonly accepted biosynthesis[17] begins with 2Z,6E-farnesyl pyrophosphate (FPP) or its allylic isomer, nerolidyl pyrophosphate. These starting materials can both produce an allylic ion that cyclises to bisabolene. Bisabolene then (after reprotonation) cyclises further to form a seven-membered ring; this may simply hydrate (forming widdrol), or it may cyclize again, forming the three membered ring system of thujopsene.
Bisabolene is also invoked as an intermediate in the related biosyntheses of juvabione, cedrene and cuparene.
Laboratory synthesis
Dauben's early synthesis of thujopsene was critical in defining the relative stereochemistry, confirming Norin's proposed cis structure.
The first laboratory total synthesis of enantiomerically pure (−)-thujopsene was by Johnson and Barbychyn in 1982, and this also used a Simmons-Smith approach, this time involving a chiral auxiliary.
Chemical and biochemical reactions
Thujopsene can easily undergo autoxidation to mayurone in high yield in the presence of a cobalt(II) dioxan complex.[18]; this conversion can also be accomplished using potassium permanganate or potassium dichromate.[7]
References
- ↑ (2009) Classics in Stereoselective Synthesis. New York: Wiley, 494. ISBN 978-3-527-29966-9. Retrieved on Aug 17, 2010.
- ↑ Norin, Torbjörn (1961). "The Chemistry of the Natural Order Cupressales. 40. The Structure of Thujopsene and Hinokiic Acid." (in English). Acta Chemica Scandinavica 15: 1676–1694. doi:10.3891/acta.chem.scand.15-1676.
- ↑ Dauben, William G.; Arnold C. Ashcraft (1963). "The Total Synthesis of (±)-Thujopsene". Journal of the American Chemical Society 85: 3673–3676. doi:10.1021/ja00905a032.
- ↑ Norin, Torbjörn (1963). "The Chemistry of the Natural Order Cupressales. 49. The Configuration of Thujopsene." (in English). Acta Chemica Scandinavica 17: 738–748. doi:10.3891/acta.chem.scand.17-0738.
- ↑ Enzell, C. (1962). "The Chemistry of the Natural Order Cupressales. 47. The Structures and Absolute Configurations of Widdrol and Widdrol-alpha-epoxide." (in English). Acta Chemica Scandinavica 16: 1553–1568. doi:10.3891/acta.chem.scand.16-1553.
- ↑ Johnson, Carl R.; Michael R. Barbachyn (1982). "β-Hydroxysulfoximine-Directed Simmons-Smith Cyclopropanations. Synthesis of (-)- and (+)-Thujopsene". Journal of the American Chemical Society 104: 4290–4291. doi:10.1021/ja00379a060.
- ↑ 7.0 7.1 Akiyoshi, Saburo; Nagahama, Shizuo (1957). "Polyterpenes I. Permanganate Oxidation of Thujopsene, Part 1" (in English). Bulletin of the Chemical Society of Japan 30: 886–889. doi:10.1246/bcsj.30.886.
- ↑ Erdtman, H.; B. R. Thomas (1958). "The Chemistry of Natural Order Cupressales. XX. Heartwood Constituents of the Genus Widdringtonia." (in English). Acta Chemica Scandinavica 12: 267–273. doi:10.3891/acta.chem.scand.12-0267.
- ↑ 9.0 9.1 Runeburg, Jarl (1960). "The Chemistry of the Natural Order Cupressales XXXI. Constituents of Juniperus phoenicea L.". Acta Chemica Scandinavica 14: 1995-1998. doi:10.3891/acta.chem.scand.14-1995.
- ↑ 10.0 10.1 Runeburg, Jarl (1960). "The Chemistry of the Natural Order Cupressales XXVIII. Constituents of Juniperus virginiana L.". Acta Chemica Scandinavica 14: 1288–1294. doi:10.3891/acta.chem.scand.14-1288.
- ↑ 11.0 11.1 {{cite journal|last=Pettersson|first=E.|coauthors=Runeburg, Jarl|year=1961|title=The Chemistry of the Natural Order Cupressales XXXIV. Constituents of Juniperus procera Hochst. and [[Juniperus californica Carr.|journal=Acta Chemica Scandinavica|volume=15|pages=713-720|doi=10.3891/acta.chem.scand.15-713|url=http://actachemscand.dk/volume.php?select1=3&vol=15}}
- ↑ Runeburg, Jarl (1961). "The Chemistry of the Natural Order Cupressales XXXIV. Constituents of Juniperus foetidissima Willd.". Acta Chemica Scandinavica 15: 721-726. doi:10.3891/acta.chem.scand.15-721.
- ↑ Pilo, Claes; Runeburg, Jarl (1960). "The Chemistry of the Natural Order Cupressales XXV. Heartwood constituents of Juniperis chinensis L.". Acta Chemica Scandinavica 14: 353–358. doi:10.3891/acta.chem.scand.14-0353.
- ↑ Runeburg, Jarl (1960). "The Chemistry of the Natural Order Cupressales XXVII. Heartwood constituents of Juniperus utahensis Lemm.". Acta Chemica Scandinavica 14: 797–804. doi:10.3891/acta.chem.scand.14-0797.
- ↑ Runeburg, Jarl (1960). "The Chemistry of the Natural Order Cupressales XXIX. Constituents of Juniperus thurifera L.". Acta Chemica Scandinavica 14: 1985–1990. doi:10.3891/acta.chem.scand.14-1985.
- ↑ Runeburg, Jarl (1960). "The Chemistry of the Natural Order Cupressales XXX. Constituents of Juniperus cedrus L.". Acta Chemica Scandinavica 14: 1991-1994. doi:10.3891/acta.chem.scand.14-1991.
- ↑ (1994) Natural Products: their chemistry and biological significance (in English). Harlow, Essex, UK: Longman, 319-320. ISBN 0-582-06009-5.
- ↑ Ito, Masaaki; Nishimura, Terumi; Abe, Kazuo (1972). "The Autocatalytic Oxidation of Thujopsene. A Facile Synthesis of Mayurone" (in English). Bulletin of the Chemical Society of Japan 45: 1914–1915. doi:10.1246/bcsj.45.1914.