Total synthesis of (-)-podophyllotoxin
Podophyllotoxin is the main active constituent in Podophyllum peltatum, also known as the American Mandrake (mayapple plant)(1). Podophyllotoxin is an extract from the plant rhizome(2). It is obtained via buffer extraction of the glucopyranoside, then converted from podophyllotoxin 4-O-β-D-glucopyranoside into the aglycone(3). It is known for its homeopathic remedies, which is a dilute form of an active ingredient (Often 0.5% solutions). Do not confuse it with a herbal remedy, which is a highly pure form of an active ingredient(1). It is a lignan which is a class of phytoestrogen (estrogen like structure)(4). An etoposide of podophyllotoxin is a candidate for attacking cancers, treating rheumatoid arthritis, psoriasis and malaria.
Contents
Natural Occurrence
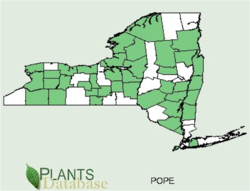
Podophyllum Peltatum occurs naturally in the Adirondacks and other various regions of New York state (green regions of map)(5). It is found in moist soils in rich woods, pastures etc. The seeds ripen July-August, and the fruits are edible, however, unripened fruit act as a strong laxative, and too much of the fruit causes colic(1).
Syntheses
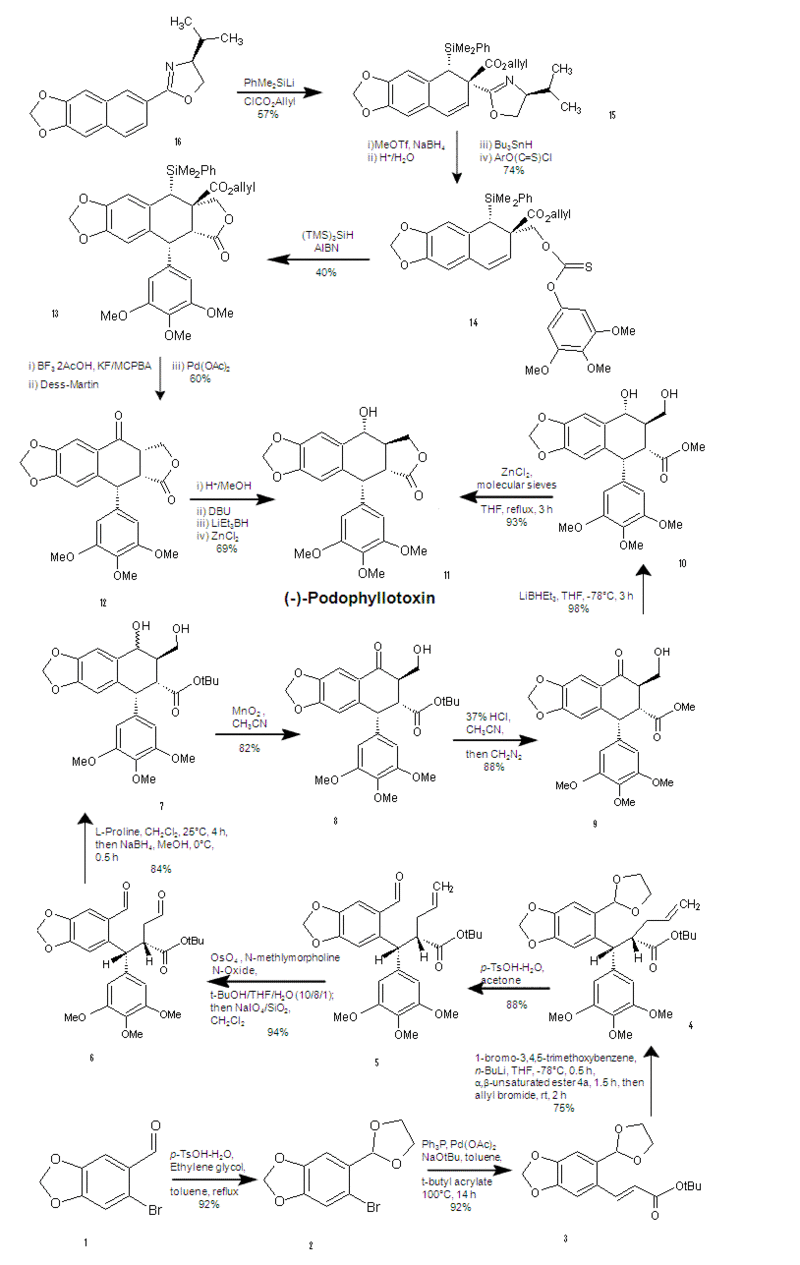
First Synthesis: Starts at the top and progresses downward to the center. Second Synthesis: Starts at the bottom and progresses upward to the center.
First Synthesis
The first step involves the addition of a large protecting group. Notice the dashed bond α to the oxazoline group. The hindered face from above forces the protecting group to attack from the bottom face. Next, the allyl ester adds from the top because of the large protecting group now hindering the bottom face. The 57% yield of this step likely results because aromaticity has to be broken. The second step involves the reduction of the oxazoline via methyl triflate and sodium borohydride. Then the dilute acid cleaves the oxazoline, while the tri-n-butyltin hydride prepares the methoxy group for the addition of the xanthate (a.k.a. thiocarbonate) group. The third step includes a free radical process in which the sillyl group bonds to the sulfur atom of the thiocarbonyl, meanwhile the carbon of the thiocarbonyl bonds to the neighboring ring, putting a radical at the benzylic position. Then the aryl transfer occurs. This step is certainly the key step, significantly preparing for the remainder of the synthesis. Being the key step, there is room for improvement with a 40% yield. The 40% yield is likely due to side reactions involving the allyl ester. The fourth step involves palladium mediated removal of the allyl ester and replacement of the PhMe2Si- group with an alcohol. This allows for Dess-Martin Periodinane (a hypervalent iodine compound)(6), reagent to oxidize the alcohol to a ketone(7). The final step of the synthesis epimerizes the C-3 position, performs stereoselective carbonyl reduction via the hindrance of DBU and forms the trans lactone (cyclic ester)(8). Overall percent yield was not provided. As mentioned above, many useful compounds are prepared from podophyllotoxin, M. Gordaliza offers a few useful procedures for obtaining these analogues(9). Multiple steps of the synthesis were performed with THF and methanol as the solvent, L. Gan et al. discovered that methanol was least able to dissolve podophyllotoxin in relation to acetone, and many other alcohols(10). Different solvents may improve yields, if they don’t provide routes for side reactions.
Second Synthesis
The first step of the alternate synthesis involves the protection of the aldehyde of 6-bromopiperonal with 1,3-dioxolane, to prepare for the heck reaction in the next step. For the heck reaction in the second step, t-butyl acrylate is used instead of methyl acrylate in order to obtain the 1,4-addition of the third step instead of the 1,2-addition which the methyl ester yields. The third step (a key step) involves halogen-metal exchange of bromine from the 1-bromo-3,4,5-trimethoxybenzene with lithium. A clever Michael addition followed, characterized by the trimethoxybenzyl group attacking the backside, then the front-face attack of the allyl group provided proper stereochemistry, pushing the t-butyl ester to the backside. The fourth step was simply the deprotection of the ketal. The fifth step utilized oxidative cleavage, then dihydroxylation via osmium tetraoxide, followed by NaIO4 yielded the dialdehyde. The sixth step (a key step) cleverly utilized L-proline to give one specific diastereomer giving the wedge bonded hydroxy group which is essential to obtaining the trans lactone in the final step. Next, to prevent dehydration by silica gel, direct reduction via sodium borohydride was performed. The seventh step involved MnO2 with acetonitrile (which was found to prevent the formation of an intramolecular hydrogen bond(11). The eighth step is a conversion to the methyl ester so methanol is the final leaving group instead of tert-butanol (an attempt to maximize yield). The ninth step utilizes super hydride to reduce the ketone, yielding the stereoselective alcohol at the C-4 position. The final step simply gave the trans lactone in high yield as in the first synthesis(11). Overall percent yield was 29%. Y. Wu et al. accomplished the synthesis of (+)-podophyllotoxin in only eight steps in 29% overall yield(12).
Uses
Podophyllotoxin is an antimitotic, which prevents cell division (confirmed in 1940)(13,14). This allows a method for treating genital warts(4). Precursor for the anti-cancer etoposide(1,2,3,13). May treat ovarian cancer, testicular cancer, leukemia, and lung cancer(1,3). New derivative (CPH 82) may treat rheumatoid arthritis(3), currently being researched. Cautions: As podophyllotoxin resin prevents mitosis; it should not be applied to open wounds or exposed areas as it prevents healing. Pregnant women should not use because it is suspect of interfering with fetal development(13). Ancient Native American uses include: Anthelmintic, which means it treats parasitic worm infections. It was used to treat snake bites and even deafness! It is used as a purgative and laxative because it is a natural irritant, also was used as a suicidal potion which is an ancient cultural practice(13).
Mode of Action
Podophyllotoxin and each of its derivatives induce a premitotic block in the late S phase or G2 phase of the cell cycle. S phase of the cell cycle is when the genetic material replicates. G2 phase is considered a growth phase, characterized by the significant increase in cell size to prepare for the formation of two daughter cells. This results from binding to topoisomerase II, which is an enzyme that is responsible for unwinding DNA during the replication process. This enzyme forms a DNA-protein link, which podophyllotoxin stabilizes, and prevents the repair of the double strand breaks(14).
References
1. Plants for a Future (2008). Podophyllum Peltatum. Plants for a Future: Edible, Medicinal, and Useful Plants for a Healthier World Retrieved 30 March 2010 from http://www.pfaf.org/database/plants.php?Podophyllum+peltatum
2. “Podophyllotoxin”. Wikipedia, The Free Encyclopedia. 28 February 2010, 23:54. Wikimedia Foundation, Inc. Retrieved 30 March, 2010 from http://en.wikipedia.org/wiki/Podophyllotoxin
3. Moraes, R.M., H. Lata, E. Bedir, M. Maqbool, and K. Cushman. 2002. The American mayapple and its potential for podophyllotoxin production. p. 527–532. In: J. Janick and A. Whipkey (eds.), Trends in new crops and new uses. ASHS Press, Alexandria, VA. Retrieved 31 March, 2010 from http://www.hort.purdue.edu/newcrop/ncnu02/v5-527.html
4. “Lignan”. Wikipedia, The Free Encyclopedia. 3 March, 2010, 14:09. Wikimedia Foundation, Inc. Retrieved 1 April, 2010 from http://en.wikipedia.org/wiki/Lignin
5. USDA. (2010). County Distribution of Podophyllum Peltatum – L. Mayapple POPE. Retrieved 31 March, 2010 from http://plants.usda.gov/java/county?state_name=NewYork&statefips=36&symbol=POPE
6. Yadav, J.S. Reddy, B.V.S. Basak, A.K. Narsaiah, A.V. (2004). Recyclable 2nd Generation Ionic Liquids as Green Solvents for the Oxidation of Alcohols with Hypervalent Iodine Reagents. Tetrahedron, 60, 2131-2135.
7. D. F. Taber. (6 September, 2004). Synthesis of (-)-Podophyllotoxin. Org. Chem. Highlights. Retrieved 24 March, 2010 from� http://www.organic-chemistry.org/Highlights/2004/06September.shtm
8. Reynolds, A.J. Scott, A.J. Turner, C.I. Sherburn, M.S. (2003). The Intramolecular Carboxyarylation Approach to Podophyllotoxin. JACS, 125, 12108-12109.
9. Gordaliza, M. (1 December, 2006). Podophyllotoxin: Sources, Extraction, and Preparation of Cytotoxic Analog Compounds. Retrieved 31 March, 2010 from http://old.iupac.org/publications/cd/medicinal_chemistry/Practica-VI-1.pdf
10. Li-She Gan, Zhen-Zhen Wang, and Chang-Xin Zhou. (2009). Solubility of Podophyllotoxin in Six Organic Solvents From (283.2 to 323.2). J. Chem. Eng. Data, 54, 160–161. Retrieved 31 March, 2010 from http://pubs.acs.org/doi/pdf/10.1021/je8007028
11. Wu, Y. Zhang, H. Zhao, Y. Zhao, J. Chen, J. Li, L. (2007). A New and Efficient Strategy for the Synthesis of Podophyllotoxin and Its Analogues. Organic Letters, 9, 1199-1202. Retrieved 31 March, 2010 from http://webproxy.potsdam.edu:2186/doi/pdf/10.1021/ol0630954
12. Wu, Y. Zhao, J. Chen, J. Pan, C. Li, L. Zhang, H. (2009). Enantioselective Sequential Conjugate Addition-Allylation Reactions: A Concise Total Synthesis of (+)-Podophyllotoxin. Organic Letters, 11, 597-600. Retrieved 31 March, 2010 from http://webproxy.potsdam.edu:2186/doi/pdf/10.1021/ol8026208
13. Ngan V. (2009). Podophyllotoxin. DermNet NZ. Retrieved 30 March, 2010 from http://www.dermnet.org.nz/treatments/podophyllotoxin.html
14. Canel, C. Moraes, R.M. Dayan, F.E. Ferreira, D. (2000). Podophyllotoxin. Phytochemistry, 54, 115-120.