Difference between revisions of "Tert-Butyl chloride"
Physchim62 (talk | contribs) (safety info) |
Physchim62 (talk | contribs) (→External links) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 23: | Line 23: | ||
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| − | | Reference = <ref name="CHRIP">{{ | + | | Reference = <ref name="CHRIP">{{CHRIP|accessdate = 2009-08-22}}.</ref> |
| Formula = C<sub>4</sub>H<sub>9</sub>Cl | | Formula = C<sub>4</sub>H<sub>9</sub>Cl | ||
| MolarMass = 92.567 g/mol | | MolarMass = 92.567 g/mol | ||
| Line 32: | Line 32: | ||
| Solubility = 0.29 g/100 ml (15 ºC) | | Solubility = 0.29 g/100 ml (15 ºC) | ||
| Solubility1 = miscible | | Solubility1 = miscible | ||
| − | | Solvent1 = | + | | Solvent1 = ethanol |
| Solubility2 = miscible | | Solubility2 = miscible | ||
| − | | Solvent2 = | + | | Solvent2 = diethyl ether |
| VaporPressure = 34.9 kPa (20 °C) | | VaporPressure = 34.9 kPa (20 °C) | ||
| HenryConstant = 0.0128 atm m<sup>3</sup>/mol (est.) | | HenryConstant = 0.0128 atm m<sup>3</sup>/mol (est.) | ||
| Line 81: | Line 81: | ||
== External links == | == External links == | ||
| − | * | + | *{{Biodegradability JP|id=1653|name=2-chloro-2-methylpropane|date=2003-01-17}} |
| − | * | + | *{{IRIS|id=0417|name=t-Butylchloride}} |
| − | * [http://wwwchem.uwimona.edu.jm:1104/lab_manuals/c10expt20.html Preparation 2-chloro-2-methylpropane] | + | * [http://wwwchem.uwimona.edu.jm:1104/lab_manuals/c10expt20.html Preparation of 2-chloro-2-methylpropane] |
* [http://www.cerlabs.com/experiments/10875407331.pdf http://www.cerlabs.com/experiments/10875407331.pdf] | * [http://www.cerlabs.com/experiments/10875407331.pdf http://www.cerlabs.com/experiments/10875407331.pdf] | ||
Latest revision as of 18:03, 24 August 2009
| tert-Butyl chloride | |
|---|---|
| IUPAC name | 2-chloro-2-methylpropane |
| Other names | 1,1-dimethylethyl chloride 1-chloro-1,1-dimethylethane chlorotrimethylmethane trimethylchloromethane t-butyl chloride |
| Identifiers | |
| InChI | InChI=1/C4H9Cl/c1-4(2,3)5/h1-3H3 |
| InChIKey | NBRKLOOSMBRFMH-UHFFFAOYAL |
| Standard InChI | InChI=1S/C4H9Cl/c1-4(2,3)5/h1-3H3 |
| Standard InChIKey | NBRKLOOSMBRFMH-UHFFFAOYSA-N |
| CAS number | [] |
| EC number | |
| UN number | 1127 |
| RTECS | TX5040000 |
| ChemSpider | |
| PubChem | |
| SMILES | |
| Properties[1] | |
| Chemical formula | C4H9Cl |
| Molar mass | 92.567 g/mol |
| Appearance | Colorless liquid |
| Density | 0.847 g/cm3 (15 ºC) |
| Melting point |
−26.5 °C |
| Boiling point |
51 °C |
| Solubility in water | 0.29 g/100 ml (15 ºC) |
| Solubility in ethanol | miscible |
| Solubility in diethyl ether | miscible |
| log P | 2.45 (est.) |
| Vapor pressure | 34.9 kPa (20 °C) |
| kH | 0.0128 atm m3/mol (est.) |
| Hazards[1][2] | |
| Material safety data sheet (MSDS) | External MSDS |
| EU index number | not listed |
| GHS pictograms | 
|
| GHS signal word | DANGER |
| GHS hazard statements | H225 |
| GHS precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+361+353, P370+378, P403+235, P501 |
| Flash point | 0 °C (32 ºF) |
| Autoignition temp. | 540 °C (1004 ºF) |
| Explosive limits | 1.8–10.1% |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
tert-Butyl chloride is a colorless, liquid organic compound at room temperature. It is sparingly soluble in water, with a tendency to undergo spontaneous solvolysis when dissolved into it. The compound is flammable and volatile, and its main use is as a starting molecule to carry out nucleophilic substitution reactions, to produce different substances, ranging from alcohols to alkoxide salts.
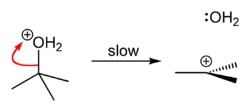
When tert-butyl chloride is dissolved in water, a polar and protic solvent, the bulky chloride substituent is carried away by it, and isolated from the aliphatic chain, causing an heterolytic rupture of the compound, giving rise to a carbocation which eventually becomes a tertiary alcohol after a water molecule reacts with it, releasing hydrochloric acid as the final product. If a different, stronger nucleophilic agent is present at the moment of reaction, reaction product may not be an alcohol, but a tertiary carbon with the nucleophile as a substituent.
Synthesis
tert-Butyl chloride can be synthesized in the laboratory by the SN1 reaction of tert-Butanol with concentrated hydrochloric acid, as shown below.
 |
 |
 |
The overall reaction, therefore, is:
Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the step 2 allows the SN1 mechanism to be followed, whereas a primary alcohol would follow an SN2 mechanism.
See also
References
- ↑ 1.0 1.1 Chemical Risk Information Platform (CHRIP), <http://www.safe.nite.go.jp/english/db.html> (accessed 22 August 2009), National Institute of Technology and Evaluation (Japan).
- ↑ HSNO Chemical Classification Information Database, <http://www.ermanz.govt.nz/Chemicals/ChemicalDisplay.aspx?SubstanceID=15657> (accessed 22 August 2009), New Zealand Environmental Risk Management Authority.
External links
- Biodegradation and Bioconcentration of 2-chloro-2-methylpropane from the National Institute of Technology and Evaluation (Japan)
- U.S. Environmental Protection Agency Integrated Risk Information System entry for "t-Butylchloride"
- Preparation of 2-chloro-2-methylpropane
- http://www.cerlabs.com/experiments/10875407331.pdf
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Tert-Butyl chloride". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |