Difference between revisions of "Alcohol"
Physchim62 (talk | contribs) (→Oxidation: correct nomenclature) |
Physchim62 (talk | contribs) (→Toxicity) |
||
| Line 189: | Line 189: | ||
An effective treatment to prevent formaldehyde toxicity after methanol ingestion is to administer ethanol. Alcohol dehydrogenase has a higher affinity for ethanol, thus preventing methanol from binding and acting as a [[substrate (biochemistry)|substrate]]. Any remaining methanol will then have time to be excreted through the kidneys. Remaining formaldehyde will be converted to [[formic acid]] and excreted.<ref>{{citation | last1 = Zimmerman | first1 = H. E. | last2 = Burkhart | first2 = K. K. | last3 = Donovan | first3 = J. W. | title = Ethylene glycol and methanol poisoning: diagnosis and treatment | journal = J. Emer. Nurs. | year = 1999 | volume = 25 | issue = 2 | pages = 116–20 | pmid = 10097201}}.</ref><ref>{{citation | last = Lobert | first = S. | title = Ethanol, isopropanol, methanol, and ethylene glycol poisoning | journal = Critical Care Nurse | year = 2000 | volume = 20 | issue = 6 | pages = 41–477 | pmid = 11878258}}.</ref> | An effective treatment to prevent formaldehyde toxicity after methanol ingestion is to administer ethanol. Alcohol dehydrogenase has a higher affinity for ethanol, thus preventing methanol from binding and acting as a [[substrate (biochemistry)|substrate]]. Any remaining methanol will then have time to be excreted through the kidneys. Remaining formaldehyde will be converted to [[formic acid]] and excreted.<ref>{{citation | last1 = Zimmerman | first1 = H. E. | last2 = Burkhart | first2 = K. K. | last3 = Donovan | first3 = J. W. | title = Ethylene glycol and methanol poisoning: diagnosis and treatment | journal = J. Emer. Nurs. | year = 1999 | volume = 25 | issue = 2 | pages = 116–20 | pmid = 10097201}}.</ref><ref>{{citation | last = Lobert | first = S. | title = Ethanol, isopropanol, methanol, and ethylene glycol poisoning | journal = Critical Care Nurse | year = 2000 | volume = 20 | issue = 6 | pages = 41–477 | pmid = 11878258}}.</ref> | ||
| − | Methanol itself, while poisonous, has a much weaker [[sedative]] effect than ethanol. Some longer-chain alcohols such as [[Propan-1-ol|''n''-propanol]], [[isopropanol]], [[Butan-1-ol|''n''-butanol]], [[Tert-Butanol|''tert''-butanol]] and [[2-methylbutan-2-ol]] (''tert''-amyl alcohol) do however have stronger sedative effects, but also have higher toxicity than ethanol. These longer chain alcohols are found as contaminants in some alcoholic beverages and are known as [[fusel alcohol]]s,<ref>{{citation | last = Woo | first = | + | Methanol itself, while poisonous, has a much weaker [[sedative]] effect than ethanol. Some longer-chain alcohols such as [[Propan-1-ol|''n''-propanol]], [[isopropanol]], [[Butan-1-ol|''n''-butanol]], [[Tert-Butanol|''tert''-butanol]] and [[2-methylbutan-2-ol]] (''tert''-amyl alcohol) do however have stronger sedative effects, but also have higher toxicity than ethanol. These longer chain alcohols are found as contaminants in some alcoholic beverages and are known as [[fusel alcohol]]s,<ref>{{citation | last = Woo | first = Kang-Lyung | title = Determination of low molecular weight alcohols including fusel oil in various samples by diethyl ether extraction and capillary gas chromatography | journal = J. AOAC Int. | year = 2005 | volume = 88 | issue = 5 | pages = 1419–27 | pmid = 16385992 | doi = 10.5555/jaoi.2005.88.5.1419}}.</ref><ref>{{citation | last1 = Lachenmeier | first1 = Dirk W. | last2 = Haupt | first2 = Simone | last3 = Schulz | first3 = Katja | title = Defining maximum levels of higher alcohols in alcoholic beverages and surrogate alcohol products | journal = Regulat. Toxicol. Pharmacol. | year = 2008 | volume = 50 | issue = 3 | pages = 313–21 | doi = 10.1016/j.yrtph.2007.12.008}}.</ref> and are reputed to cause severe [[hangover]]s although it is unclear if the fusel alcohols are actually responsible.<ref>{{citation | last1 = Hori | first1 = Hisako | last2 = Fujii | first2 = Wataru | last3 = Hatanaka | first3 = Yutaka | last4 = Suwa | first4 = Yoshihide | title = Effects of fusel oil on animal hangover models | journal = Alcohol Clin. Exp. Res. | year = 2003 | volume = 27 | issue = 8 Suppl | pages = 37S–41S | doi = 10.1097/01.ALC.0000078828.49740.48}}.</ref> Many longer chain alcohols are used in industry as [[solvent]]s and are occasionally abused by [[alcoholic]]s, leading to a range of adverse health effects.<ref>{{citation | last1 = McKee | first1 = Martin | last2 = Sűzcs | first2 = Sándor | last3 = Sárváry | first3 = Attila | last4 = Adany | first4 = Roza | last5 = Kiryanov | first5 = Nikolay | last6 = Saburova | first6 = Ludmilla | last7 = Tomkins | first7 = Susannah | last8 = Andreev | first8 = Evgeny | last9 = Leon | first9 = David A. | title = The composition of surrogate alcohols consumed in Russia | journal = Alcohol. Clin. Exp. Res. | year = 2005 | volume = 29 | issue = 10 | pages = 1884–88 | doi = 10.1097/01.alc.0000183012.93303.90}}.</ref><ref>{{citation | last1 = Bunc | first1 = M. | last2 = Pezdir | first2 = T. | last3 = Možina | first3 = H. | last4 = Možina | first4 = M. | last5 = Brvar | first5 = M. | title = Butanol ingestion in an airport hangar | journal = Hum. Exp. Toxicol. | year = 2006 | volume = 25 | issue = 4 | page = 195–97 | doi = 10.1191/0960327106ht607oa}}.</ref> |
==Occurrence in nature== | ==Occurrence in nature== | ||
Revision as of 16:53, 30 August 2009
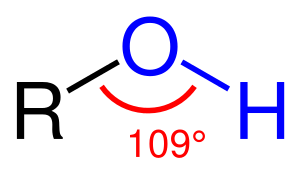
An alcohol is any organic compound in which a hydroxyl group (–OH) is bound to a saturated carbon atom,[1][2] that is the carbon atom of an alkyl or substituted alkyl group. The general formula for a simple acyclic alcohol is CnH2n+1OH. In common terms, the word "alcohol" refers to ethanol, the type of alcohol found in alcoholic beverages.
Ethanol is a colorless, volatile liquid with a mild odor which can be obtained by the fermentation of sugars. (Industrially, it is more commonly obtained by ethylene hydration—the reaction of ethylene with water in the presence of phosphoric acid.[3]) Ethanol is the most widely used depressant in the world, and has been for thousands of years. This sense underlies the term alcoholism (addiction to alcohol).
There are three major subsets of alcohols: primary (1°), secondary (2°) and tertiary (3°), based upon the number of carbon atoms the C-OH group's carbon (shown in red) is bonded to. Ethanol is a simple 'primary' alcohol. The simplest secondary alcohol is isopropyl alcohol (propan-2-ol), and the simplest tertiary alcohol is tert-butyl alcohol (2-methylpropan-2-ol).
Contents
Simple alcohols
The simplest and most commonly used alcohols are methanol and ethanol. Methanol was formerly obtained by the distillation of wood and called "wood alcohol."
One sip of methanol (as little as 10ml) can cause permanent blindness by destruction of the optic nerve.[4] Apart from its familiar role in alcoholic beverages, ethanol is also used as a highly controlled industrial solvent and raw material. To avoid the high taxes on ethanol for consumption, additives are added to make it unpalatable (such as denatonium benzoate—"Bitrex") or poisonous (such as methanol). Ethanol in this form is known generally as denatured alcohol; when methanol is used, it may be referred to as methylated spirits ("Meths") or "surgical spirits".
Two other alcohols whose uses are relatively widespread (though not so much as those of methanol and ethanol) are propanol and butanol. Like ethanol, they can be produced by fermentation processes. (However, the fermenting agent is a bacterium, Clostridium acetobutylicum, that feeds on cellulose, not sugars like the Saccharomyces yeast that produces ethanol.)
Nomenclature
In the IUPAC system, the name of the alkane chain loses the terminal "e" and adds "ol", e.g. "methanol" and "ethanol".[5] When necessary, the position of the hydroxyl group is indicated by a number between the alkane name and the "ol": propan-1-ol for CH3CH2CH2OH, propan-2-ol for CH3CH(OH)CH3. In CAS nomenclature, widely used in the United States, the position number is written before the name: 1-propanol and 2-propanol. If a higher priority group is present (such as an aldehyde, ketone or carboxylic acid), then it is necessary to use the prefix "hydroxy",[5] for example: 1-hydroxy-2-propanone (CH3COCH2OH).
Some examples of simple alcohols and how to name them:
Common names for alcohols usually takes name of the corresponding alkyl group and add the word "alcohol", e.g. methyl alcohol, ethyl alcohol or tert-butyl alcohol. Propyl alcohol may be n-propyl alcohol or isopropyl alcohol depending on whether the hydroxyl group is bonded to the 1st or 2nd carbon on the propane chain. Isopropyl alcohol is also occasionally called sec-propyl alcohol.
As mentioned above alcohols are classified as primary (1°), secondary (2°) or tertiary (3°), and common names often indicate this in the alkyl group prefix. For example (CH3)3COH is a tertiary alcohol is commonly known as tert-butyl alcohol. This would be named 2-methylpropan-2-ol under IUPAC rules, indicating a propane chain with methyl and hydroxyl groups both attached to the middle (#2) carbon.
Primary alcohol (1°)- Have general formulas RCH2OH Secondary alcohol (2°)- Have general formulas RR'CHOH Tertiary alcohol (3°)- Have general formulas RR'RCOH Hydrogen bond strength order: 1°>2°>3° Boiling point order: 1°>2°>3° Acidity order: 1°>2°>3°
The definition of an alcohol is specific that the hydroxy group must be attached to a saturated carbon atom.[1][2] When the hydroxy group is attached to an aromatic ring, the functional group is called a phenol:[6] the compound named phenol is the simplest example of this class of compounds. When the hydroxy group is attached to an sp2 carbon atom which is not part of an aromatic ring, the functional group is called an enol:[7] enols are generally unstable with respect to tautomerism to aldehydes or ketones, but can be very important as reactive intermediates in organic reactions.
Etymology
The word alcohol appears in English in the 16th century, loaned via French from medical Latin, ultimately from the Arabic الكحل (al-kuḥl, "the kohl, a powder used as an eyeliner").
ال al is Arabic for the definitive article, the in English.
The current Arabic name for alcohol is الكحول al-kuḥūl, re-introduced from western usage.
kuḥl was the name given to the very fine powder, produced by the sublimation of the natural mineral stibnite to form antimony sulfide Sb2S3 (hence the essence or "spirit" of the substance), which was used as an antiseptic and eyeliner.
Bartholomew Traheron in his 1543 translation of John of Vigo introduces the word as a term used by "barbarous" (Moorish) authors for "fine powder":
- the barbarous auctours use alcohol, or (as I fynde it sometymes wryten) alcofoll, for moost fine poudre.
William Johnson in his 1657 Lexicon Chymicum glosses the word as antimonium sive stibium. By extension, the word came to refer to any fluid obtained by distillation, including "alcohol of wine", the distilled essence of wine. Libavius in Alchymia (1594) has vini alcohol vel vinum alcalisatum. Johnson (1657) glosses alcohol vini as quando omnis superfluitas vini a vino separatur, ita ut accensum ardeat donec totum consumatur, nihilque fæcum aut phlegmatis in fundo remaneat. The word's meaning became restricted to "spirit of wine" (ethanol) in the 18th century, and was again extended to the family of substances so called in modern chemistry from 1850.
Physical and chemical properties
Alcohols have an odor that is often described as “biting” and as “hanging” in the nasal passages.
The hydroxyl group generally makes the alcohol molecule polar. Those groups can form hydrogen bonds to one another and to other compounds. This hydrogen bonding means that alcohols can be used as protic solvents. Two opposing solubility trends in alcohols are: the tendency of the polar OH to promote solubility in water, and of the carbon chain to resist it. Thus, methanol, ethanol, and propanol are miscible in water because the hydroxyl group wins out over the short carbon chain. Butanol, with a four-carbon chain, is moderately soluble because of a balance between the two trends. Alcohols of five or more carbons (Pentanol and higher) are effectively insoluble in water because of the hydrocarbon chain's dominance. All simple alcohols are miscible in organic solvents.
Because of hydrogen bonding, alcohols tend to have higher boiling points than comparable hydrocarbons and ethers. The boiling point of the alcohol ethanol is 78.29 °C, compared to 69 °C for the hydrocarbon hexane (a common constituent of gasoline), and 34.6 °C for diethyl ether.
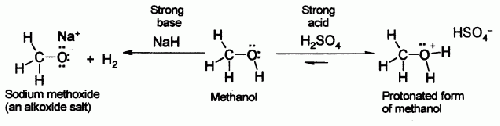
Alcohols, like water, can show either acidic or basic properties at the O-H group. With a pKa of around 16–19 they are generally slightly weaker acids than water, but they are still able to react with strong bases such as sodium hydride or reactive metals such as sodium. The salts that result are called alkoxides, with the general formula RO-M+.
Meanwhile the oxygen atom has lone pairs of nonbonded electrons that render it weakly basic in the presence of strong acids such as sulfuric acid. For example, with methanol:
Alcohols can also undergo oxidation to give aldehydes, ketones or carboxylic acids, or they can be dehydrated to alkenes. They can react to form ester compounds, and they can (if activated first) undergo nucleophilic substitution reactions. The lone pairs of electrons on the oxygen of the hydroxyl group also makes alcohols nucleophiles. For more details see the reactions of alcohols section below.
Applications
Alcohols can be used as a beverage (ethanol only), as fuel and for many scientific, medical, and industrial utilities. Ethanol in the form of alcoholic beverages has been consumed by humans since pre-historic times. A 50% v/v solution of ethylene glycol in water is commonly used as an antifreeze.
Some alcohols, mainly ethanol and methanol, can be used as an alcohol fuel. Fuel performance can be increased in forced induction internal combustion engines by injecting alcohol into the air intake after the turbocharger or supercharger has pressurized the air. This cools the pressurized air, providing a denser air charge, which allows for more fuel, and therefore more power.
Alcohols have applications in industry and science as reagents or solvents. Because of its low toxicity and ability to dissolve non-polar substances, ethanol can be used as a solvent in medical drugs, perfumes, and vegetable essences such as vanilla. In organic synthesis, alcohols serve as versatile intermediates.
Ethanol can be used as an antiseptic to disinfect the skin before injections are given, often along with iodine. Ethanol-based soaps are becoming common in restaurants and are convenient because they do not require drying due to the volatility of the compound. Alcohol is also used as a preservative for specimens.
Alcohol gels have become common as hand sanitizers.
Production
Industrially alcohols are produced in several ways:
- By fermentation using glucose produced from sugar from the hydrolysis of starch, in the presence of yeast and temperature of less than 37°C to produce ethanol. For instance the conversion of invertase to glucose and fructose or the conversion of glucose to zymase and ethanol.
- By direct hydration using ethylene (ethylene hydration[3] or other alkenes from cracking of fractions of distilled crude oil.
Endogenous
It is inevitable that all humans always have some amount of alcohol in their bodies at all times, even if they never drink alcoholic beverages in their lives. This is because of a process called endogenous ethanol production. Many of the bacteria in the intestines use alcohol fermentation as a form of respiration. This metabolic method produces alcohol as a waste product, in the same way that metabolism results in the formation of carbon dioxide and water. Thus, human bodies always contain some quantity of alcohol produced by these benign bacteria.
Laboratory synthesis
Several methods exist for the preparation of alcohols in the laboratory.
- Primary alkyl halides react with aqueous NaOH or KOH mainly to primary alcohols in nucleophilic aliphatic substitution. (Secondary and especially tertiary alkyl halides will give the elimination (alkene) product instead).
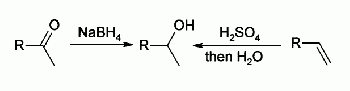
- Aldehydes or ketones are reduced with sodium borohydride or lithium aluminium hydride (after an acidic workup). Another reduction by aluminiumisopropylates is the Meerwein-Ponndorf-Verley reduction.
- Alkenes engage in an acid catalysed hydration reaction using concentrated sulfuric acid as a catalyst which gives usually secondary or tertiary alcohols. The hydroboration-oxidation and oxymercuration-reduction of alkenes are more reliable in organic synthesis. Alkenes react with NBS and water in halohydrin formation reaction
- Grignard reagents react with carbonyl groups to secondary and tertiary alcohols. Related reactions are the Barbier reaction and the Nozaki-Hiyama reaction.
- Noyori asymmetric hydrogenation is the asymmetric reduction of β-keto-esters
- Amines can be converted to diazonium salts which are then hydrolyzed.
The formation of a secondary alcohol via reduction and hydration is shown:
Reactions
Deprotonation
Alcohols can behave as weak acids, undergoing deprotonation. The deprotonation reaction to produce an alkoxide salt is either performed with a strong base such as sodium hydride or n-butyllithium, or with sodium or potassium metal.
- 2R–OH + 2NaH → 2R–O-Na+ + 2H2 ↑
- 2R–OH + 2Na → 2R–O−Na+ + H2 ↑
- E.g. 2CH3CH2–OH + 2Na → 2CH3-CH2-O−Na+ + H2 ↑
Water is similar in pKa to many alcohols, so with sodium hydroxide there is an equilibrium set up which usually lies to the left:
- R–OH + NaOH ⇌ R–O-Na+ + H2O (equilibrium to the left)
It should be noted, though, that the bases used to deprotonate alcohols are strong themselves. The bases used and the alkoxides created are both highly moisture sensitive chemical reagents.
The acidity of alcohols is also affected by the overall stability of the alkoxide ion. Electron-withdrawing groups attached to the carbon containing the hydroxyl group will serve to stabilize the alkoxide when formed, thus resulting in greater acidity. On the other hand, the presence of electron-donating group will result in a less stable alkoxide ion formed. This will result in a scenario whereby the unstable alkoxide ion formed will tend to accept a proton to reform the original alcohol.
With alkyl halides alkoxides give rise to ethers in the Williamson ether synthesis.
Nucleophilic substitution
The OH group is not a good leaving group in nucleophilic substitution reactions, so neutral alcohols do not react in such reactions. However, if the oxygen is first protonated to give R–OH2+, the leaving group (water) is much more stable, and the nucleophilic substitution can take place. For instance, tertiary alcohols react with hydrochloric acid to produce tertiary alkyl halides, where the hydroxyl group is replaced by a chlorine atom by unimolecular nucleophilic substitution. If primary or secondary alcohols are to be reacted with hydrochloric acid, an activator such as zinc chloride is needed. Alternatively the conversion may be performed directly using thionyl chloride.
Alcohols may likewise be converted to alkyl bromides using hydrobromic acid or phosphorus tribromide, for example:
- 3R–OH + PBr3 → 3 RBr + H3PO3
In the Barton–McCombie deoxygenation an alcohol is deoxygenated to an alkane with tributyltin hydride or a trimethylborane–water complex in a radical substitution reaction.
Dehydration
Alcohols are themselves nucleophilic, so R–OH2+ can react with ROH to produce ethers and water in a dehydration reaction, although this reaction is rarely used except in the manufacture of diethyl ether.
More useful is the E1 elimination reaction of alcohols to produce alkenes. The reaction generally obeys Zaitsev's Rule, which states that the most stable (usually the most substituted) alkene is formed. Tertiary alcohols eliminate easily at just above room temperature, but primary alcohols require a higher temperature.
This is a diagram of acid catalysed dehydration of ethanol to produce ethene:
A more controlled elimination reaction is the Chugaev elimination with carbon disulfide and iodomethane.
Esterification
To form an ester from an alcohol and a carboxylic acid the reaction, known as Fischer esterification, is usually performed at reflux with a catalyst of concentrated sulfuric acid:
- R–OH + R'–COOH ⇌ R'–COOR + H2O
In order to drive the equilibrium to the right and produce a good yield of ester, water is usually removed, either by an excess of H2SO4 or by using a Dean–Stark apparatus. Esters may also be prepared by reaction of the alcohol with an acid chloride in the presence of a base such as pyridine.
Other types of ester are prepared similarly: for example tosyl (tosylate) esters are made by reaction of the alcohol with p-toluenesulfonyl chloride in pyridine.
Oxidation
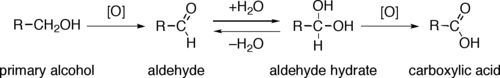
Primary alcohols (R–CH2–OH) can be oxidized either to aldehydes (R–CHO) or to carboxylic acids (R–CO2H), while the oxidation of secondary alcohols (R1R2CH–OH) normally terminates at the ketone (R1R2C=O) stage. Tertiary alcohols (R1R2R3C–OH) are resistant to oxidation.
The direct oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an acetal (R–CH(OH)2, also known as a gem-diol) by reaction with water before it can be further oxidized to the carboxylic acid.
Often it is possible to interrupt the oxidation of a primary alcohol at the aldehyde level by performing the reaction in absence of water, so that no aldehyde hydrate can be formed.
Reagents useful for the transformation of primary alcohols to aldehydes are normally also suitable for the oxidation of secondary alcohols to ketones. These include:
- Chromium-based reagents, such as Collins reagent (CrO3·Py2), pyridinium dichromate (PDC) or pyridinium chlorochromate (PCC).
- Activated dimethyl sulfoxide (DMSO), resulting from reaction of DMSO with an electrophile such as oxalyl chloride (Swern oxidation), a carbodiimide (Pfitzner–Moffatt oxidation) or the complex SO3·Py (Parikh–Doering oxidation).
- Hypervalent iodine compounds, such as Dess–Martin periodinane or 2-iodoxybenzoic acid.
- Catalytic Tetrapropylammonium perruthenate (TPAP) in presence of excess of N-methylmorpholine N-oxide (NMO) (Ley oxidation).
- Catalytic TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl radical) in presence of excess bleach (NaOCl) (Anelli's oxidation).
Allylic and benzylic alcohols can be oxidized in presence of other alcohols using certain selective oxidants such as manganese dioxide (MnO2).
Reagents useful for the oxidation of secondary alcohols to ketones, but normally inefficient for oxidation of primary alcohols to aldehydes, include chromium trioxide (CrO3) in a mixture of sulfuric acid and acetone (Jones oxidation) and certain ketones, such as cyclohexanone, in the presence of aluminium isopropoxide (Oppenauer oxidation).
The direct oxidation of primary alcohols to carboxylic acids can be carried out using:
- Potassium permanganate (KMnO4).
- Jones oxidation.
- Pyridinium dichromate (PDC) in 2,5-dimethylfuran.
- Heyns oxidation.
- Ruthenium tetroxide (RuO4).
- TEMPO.
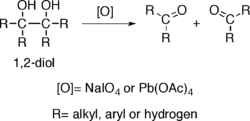
Alcohols possessing two hydroxy groups located on adjacent carbons —that is, 1,2-diols— suffer oxidative breakage at a carbon-carbon bond with some oxidants such as sodium periodate (NaIO4) or lead tetraacetate (Pb(OAc)4), resulting in generation of two carbonyl groups.
Toxicity

Ethanol in alcoholic beverages has been consumed by humans since prehistoric times for a variety of hygienic, dietary, medicinal, religious, and recreational reasons. The consumption of large doses of ethanol causes drunkenness (intoxication), which may lead to a hangover as its effects wear off. Depending upon the dose and the regularity of its consumption, ethanol can cause acute respiratory failure or death. Because ethanol impairs judgment in humans, it can be a catalyst for reckless or irresponsible behavior.
The LD50 of ethanol in rats is 10,300 mg/kg.[9] Other alcohols are substantially more poisonous than ethanol, partly because they take much longer to be metabolized and partly because their metabolization produces substances that are even more toxic. Methanol (wood alcohol), for instance, is oxidized to the poisonous formaldehyde in the liver by alcohol dehydrogenase enzymes; this can cause blindness or death.[4]
An effective treatment to prevent formaldehyde toxicity after methanol ingestion is to administer ethanol. Alcohol dehydrogenase has a higher affinity for ethanol, thus preventing methanol from binding and acting as a substrate. Any remaining methanol will then have time to be excreted through the kidneys. Remaining formaldehyde will be converted to formic acid and excreted.[10][11]
Methanol itself, while poisonous, has a much weaker sedative effect than ethanol. Some longer-chain alcohols such as n-propanol, isopropanol, n-butanol, tert-butanol and 2-methylbutan-2-ol (tert-amyl alcohol) do however have stronger sedative effects, but also have higher toxicity than ethanol. These longer chain alcohols are found as contaminants in some alcoholic beverages and are known as fusel alcohols,[12][13] and are reputed to cause severe hangovers although it is unclear if the fusel alcohols are actually responsible.[14] Many longer chain alcohols are used in industry as solvents and are occasionally abused by alcoholics, leading to a range of adverse health effects.[15][16]
Occurrence in nature
Alcohol has been found outside the Solar system. It can be found in low densities in star and planetary system forming regions of space.[17][18]
References
- ↑ 1.0 1.1 Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67 (8-9), 1307–75 at 1312. DOI: 10.1351/pac199567081307.
- ↑ 2.0 2.1 alcohols, <http://goldbook.iupac.org/A00204.html> (accessed 24 August 2009), Compendium of Chemical Terminology Internet edition; International Union of Pure and Applied Chemistry (IUPAC).
- ↑ 3.0 3.1 Lodgsdon, J. E. Ethanol. In Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; Kroschwitz, J. I.; Howe-Grant, M., Eds.; John Wiley: New York, 1994; Vol. 9, p 820.
- ↑ 4.0 4.1 Methanol and Blindness; Ask A Scientist: Chemistry Archive; U.S. Department of Energy, May 2005, <http://www.newton.dep.anl.gov/askasci/chem03/chem03561.htm>. (accessed 22 May 2007).
- ↑ 5.0 5.1 Reusch, William Alcohols, <http://www.cem.msu.edu/~reusch/VirtualText/alcohol1.htm#alcnom> (accessed 14 September 2007), VirtualText of Organic Chemistry; Department of Chemistry, Michigan State University.
- ↑ phenols, <http://goldbook.iupac.org/P04539.html> (accessed 24 August 2009), Compendium of Chemical Terminology Internet edition; International Union of Pure and Applied Chemistry (IUPAC).
- ↑ enols, <http://goldbook.iupac.org/E02124.html> (accessed 24 August 2009), Compendium of Chemical Terminology Internet edition; International Union of Pure and Applied Chemistry (IUPAC).
- ↑ Global Status Report on Alcohol 2004; World Health Organization, 2004, <http://www.who.int/entity/substance_abuse/publications/global_status_report_2004_overview.pdf>.
- ↑ Gable, Robert S. Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction 2004, 99 (6), 686–96. DOI: 10.1111/j.1360-0443.2004.00744.x.
- ↑ Zimmerman, H. E.; Burkhart, K. K.; Donovan, J. W. Ethylene glycol and methanol poisoning: diagnosis and treatment. J. Emer. Nurs. 1999, 25 (2), 116–20. PMID 10097201.
- ↑ Lobert, S. Ethanol, isopropanol, methanol, and ethylene glycol poisoning. Critical Care Nurse 2000, 20 (6), 41–477. PMID 11878258.
- ↑ Woo, Kang-Lyung Determination of low molecular weight alcohols including fusel oil in various samples by diethyl ether extraction and capillary gas chromatography. J. AOAC Int. 2005, 88 (5), 1419–27. PMID 16385992. DOI: 10.5555/jaoi.2005.88.5.1419.
- ↑ Lachenmeier, Dirk W.; Haupt, Simone; Schulz, Katja Defining maximum levels of higher alcohols in alcoholic beverages and surrogate alcohol products. Regulat. Toxicol. Pharmacol. 2008, 50 (3), 313–21. DOI: 10.1016/j.yrtph.2007.12.008.
- ↑ Hori, Hisako; Fujii, Wataru; Hatanaka, Yutaka; Suwa, Yoshihide Effects of fusel oil on animal hangover models. Alcohol Clin. Exp. Res. 2003, 27 (8 Suppl), 37S–41S. DOI: 10.1097/01.ALC.0000078828.49740.48.
- ↑ McKee, Martin; Sűzcs, Sándor; Sárváry, Attila; Adany, Roza; Kiryanov, Nikolay; Saburova, Ludmilla; Tomkins, Susannah; Andreev, Evgeny, et al. The composition of surrogate alcohols consumed in Russia. Alcohol. Clin. Exp. Res. 2005, 29 (10), 1884–88. DOI: 10.1097/01.alc.0000183012.93303.90.
- ↑ Bunc, M.; Pezdir, T.; Možina, H.; Možina, M.; Brvar, M. Butanol ingestion in an airport hangar. Hum. Exp. Toxicol. 2006, 25 (4), 195–97. DOI: 10.1191/0960327106ht607oa.
- ↑ Buhl, David Galactic clouds of organic molecules. Orig. Life Evol. Biospheres 1794, 5 (1-2), 29–40. DOI: 10.1007/BF00927011.
- ↑ Ross, John E. The Interstellar Medium, <http://www.physics.uq.edu.au/people/ross/phys2080/ism/ism.htm> (accessed 24 August 2009), Department of Physics, University of Queensland, August 1998.
Further reading
- Metcalf, Allan A. The World in So Many Words; Houghton Mifflin, 1999. ISBN 0395959209, <http://books.google.ca/books?id=4O0W5XyQVCYC&pg=PA123&dq=sash+etymology+arabic&lr=&as_brr=3&sig=iuzjUzyPphZKCIJLAwJZE7beIEI#PPA123,M1>.
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Alcohol". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |