Difference between revisions of "Isopropanol"
Physchim62 (talk | contribs) (Imported from http://en.wikipedia.org/w/index.php?title=Isopropyl_alcohol&oldid=307833067) |
Physchim62 (talk | contribs) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{chembox | {{chembox | ||
| − | | | + | | Name = Isopropanol |
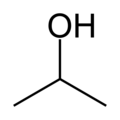
| − | | | + | | ImageFileL1 = isopropanol-skeletal.png |
| ImageSizeL1 = 100px | | ImageSizeL1 = 100px | ||
| ImageNameL1 = isopropyl alcohol | | ImageNameL1 = isopropyl alcohol | ||
| − | | | + | | ImageFileR1 = isopropanol-3D-vdW.png |
| ImageSizeR1 = 120px | | ImageSizeR1 = 120px | ||
| ImageNameR1 = isopropanol | | ImageNameR1 = isopropanol | ||
| − | | | + | | IUPACName = propan-2-ol |
| − | | OtherNames = 2- | + | | OtherNames = 2-Propanol<br/>Isopropyl alcohol |
| Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| − | | | + | | InChI = 1/C3H8O/c1-3(2)4/h3-4H,1-2H3 |
| + | | StdInChI = 1S/C3H8O/c1-3(2)4/h3-4H,1-2H3 | ||
| + | | InChIKey = KFZMGEQAYNKOFK-UHFFFAOYAH | ||
| + | | StdInChIKey = KFZMGEQAYNKOFK-UHFFFAOYSA-N | ||
| CASNo = 67-63-0 | | CASNo = 67-63-0 | ||
| CASNo_Ref = {{cascite}} | | CASNo_Ref = {{cascite}} | ||
| + | | EC-number = 200-661-7 | ||
| ChemSpiderID = 3644 | | ChemSpiderID = 3644 | ||
| RTECS = NT8050000 | | RTECS = NT8050000 | ||
| + | | SMILES = CC(O)C | ||
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| + | | Reference = <ref name="ICSC">{{ICSC-ref|0554|name=Isopropyl alcohol|date=March 1999}}.</ref><ref name="Yaws">{{citation | title = Chemical Properties Handbook: Physical, Thermodynamic, Environmental, Transport, Safety, and Health Related Properties for Organic and Inorganic Chemicals | last = Yaws | first = Carl L. | publisher = McGraw-Hill | location = New York | year = 1998 | isbn = 0070734011}}.</ref> | ||
| Formula = C<sub>3</sub>H<sub>7</sub>OH | | Formula = C<sub>3</sub>H<sub>7</sub>OH | ||
| − | | MolarMass = 60. | + | | MolarMass = 60.095 g/mol |
| − | | Appearance = | + | | Appearance = colorless liquid |
| − | | Density = | + | | Density = 0.786 g/cm<sup>3</sup> (20 ºC), liquid |
| − | | Solubility = | + | | Solubility = miscible |
| − | | MeltingPtC = | + | | MeltingPtC = −90 |
| BoilingPtC = 82.3 | | BoilingPtC = 82.3 | ||
| − | | pKa = 16.5 | + | | pKa = 16.5 |
| − | | Viscosity = | + | | Viscosity = 1.96 cP (25 °C) |
| − | | | + | | Dipole = 1.66 D (gas) |
| + | | LogP = 0.05 | ||
| + | | VaporPressure = 4.4 kPa (20 ºC) | ||
| + | | LambdaMax = 204 nm | ||
}} | }} | ||
| Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| − | | | + | | Reference = <ref name="ICSC"/><ref>{{CLP Regulation|index=603-117-00-0|page=493}}</ref><ref>{{PGCH-ref|0359|name=Isopropyl alcohol}}.</ref> |
| − | | | + | | ExternalMSDS = {{ICSC-small|0554}} |
| + | | EUIndex = 603-117-00-0 | ||
| + | | GHSPictograms = {{GHS02|Flam. Liq.2}}{{GHS07|Eye Irrit. 2; STOT SE 3}} | ||
| + | | GHSSignalWord = DANGER | ||
| + | | HPhrases = {{H-phrases|225|319|336}} | ||
| FlashPt = 12 °C | | FlashPt = 12 °C | ||
| − | | | + | | Autoignition = 456 ºC |
| − | | | + | | ExploLimits = 2–12% |
| − | | | + | | PEL = 400 ppm TWA |
| − | |||
| − | |||
}} | }} | ||
| Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| − | | | + | | OtherFunctn = [[Ethanol]]<br/>[[Propan-1-ol]]<br/>[[Butan-2-ol]] |
| − | + | | Function = [[alcohol]]s | |
| + | | OtherCpds = [[Acetone]] | ||
}} | }} | ||
}} | }} | ||
| − | ''' | + | '''Isopropanol''', also known as '''isopropyl alcohol''', '''propan-2-ol''', '''2-propanol''', '''iso''', '''isopro''', '''isoprop''', '''[[rubbing alcohol]]''', or the abbreviation '''IPA''', is a [[colorless]], [[flammable]] [[chemical compound]] with a strong [[odor]]. It has the [[molecular formula]] C<sub>3</sub>H<sub>7</sub>OH and is the simplest example of a [[Alcohol#Primary.2C secondary.2C and tertiary alcohols|''secondary alcohol'']], where the [[alcohol]] carbon is attached to two other carbons. It is an [[isomer]] of [[propanol]]. |
==Production== | ==Production== | ||
| + | Isopropanol is produced by combining water and [[propene]].<ref>{{Kirk-Othmer | contribution = Isopropyl Alcohol | first1 = John E. | last1 = Logsdon | first2 = Richard A. | last2 = Loke | accessdate = 2007-08-30}}.</ref> There are two processes for achieving this: indirect [[hydration reaction|hydration]] via the sulfuric acid process and direct hydration. The former process, which can use low-quality propylene, predominates in the USA while the latter process, which requires high-purity propylene, is more commonly used in Europe. These processes give predominantly isopropyl alcohol rather than propan-1-ol because the addition of water or [[sulfuric acid]] to propylene follows [[Markovnikov's rule]]. | ||
| − | + | The indirect process reacts propylene with sulfuric acid to form a mixture of sulfate esters. Subsequent [[hydrolysis]] of these esters produces isopropanol. Direct hydration reacts propylene and water, either in gas or liquid phases, at high pressures in the presence of solid or supported acidic [[catalyst]]s. Both processes require that the isopropanol be separated from water and other by-products by [[distillation]]. Isopropanol and water form an [[azeotrope]] and simple distillation gives a material which is 87.9% by weight isopropyl alcohol and 12.1% by weight water.<ref>{{RubberBible44th|pages=2143–84}}.</ref> Pure (anhydrous) isopropanol is made by [[azeotropic distillation]] of the "wet" isopropyl alcohol using either [[diisopropyl ether]] or [[cyclohexane]] as azeotroping agents. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | The indirect process reacts propylene with sulfuric acid to form a mixture of sulfate esters. Subsequent [[hydrolysis]] of these esters produces | ||
==Uses== | ==Uses== | ||
| − | + | Isopropanol is cheaply available. Like [[acetone]], it dissolves a wide range of [[nonpolar]] compounds. It is also relatively nontoxic and dries (evaporates) quickly. Thus it is used widely as a solvent and as a cleaning fluid, especially for dissolving [[lipophilic]] contaminants such as oil. Examples of this use include cleaning [[electronics|electronic]] devices such as contact pins (like those on [[read-only memory|ROM]] [[cartridge (electronics)|cartridges]]), [[magnetic tape]] and disk heads (such as those in audio and video tape recorders and [[floppy disk]] drives), the lenses of [[laser]]s in [[optical disc]] drives (e.g. [[CD]], [[DVD]]) and removing [[thermal paste]] from [[IC]] packages (such as [[central processing unit|CPUs]].) It is also used to clean [[liquid crystal display|LCD]] and glass [[Visual display unit|computer monitor]] screens (at some risk to the anti-reflection coating of some screens), and used by many music shops to give second-hand or worn [[Gramophone record|records]] newer-looking sheens (though it may leach [[plasticizer]] from [[vinyl]], making it more rigid). Pure isopropanol should not be used to clean vinyl records. A basic reciepe for cleaning vinyl records is: for 1 litre, 50% isopropanol, 50% demineralised water, one or two drops of pure detergent such as Kodak PhotoFlo. It cleans white boards and other unwanted ink related marks very well (at the risk of damaging the non-stick surface of the white board). Isopropanol also works well at removing smudges, dirt, and fingerprints from [[cell phones]] and [[PDA]]s. It is effective at removing residual glue from some sticky labels (but some other adhesives used on tapes and paper labels are resistant to it.) It can also be used to remove stains from most fabrics, wood, cotton, etc. Isopropanol is also used to remove [[brake fluid]] traces from hydraulic [[disk brake]] systems, so that the brake fluid (usually [[DOT 3]], [[DOT 4]] or [[mineral oil]]) does not contaminate the [[brake pads]], which would result in poor braking. In addition it can also be used to clean paintballs or other oil based products so that they may be reused, commonly known as "repainting". | |
| − | As a preservative (for biological specimens) | + | As a preservative (for biological specimens) isopropanol provides a cost-effective (when compared to pure [[ethanol]]) and comparatively non-toxic alternative to [[formaldehyde]] and other synthetic preservatives. When used for the preservation of specimens in solution concentrations of 90–99% are optimal, though concentrations as low as 70% can be used in emergencies. |
| − | [[Sterilization (microbiology)|Sterilizing]] pads typically contain a 60–70% [[solution]] of isopropanol in [[water (molecule)|water]]. | + | [[Sterilization (microbiology)|Sterilizing]] pads typically contain a 60–70% [[solution]] of isopropanol in [[water (molecule)|water]]. Isopropanol is also commonly used as a [[cleaner]] and [[solvent]] in industry. |
Isopropanol is a major ingredient in "gas dryer" [[fuel additive]]s. In significant quantities, [[water]] is a problem in fuel tanks, as it separates from the gasoline, and can freeze in the supply lines at cold temperatures. The isopropanol does not remove the water from the gasoline; rather, the isopropanol solubilizes the water in the gasoline. Once [[soluble]], the water does not pose the same risk as insoluble water as it will no longer accumulate in the supply lines and freeze. Isopropanol is often sold in aerosol cans as a [[windshield|windscreen]] de-icer. | Isopropanol is a major ingredient in "gas dryer" [[fuel additive]]s. In significant quantities, [[water]] is a problem in fuel tanks, as it separates from the gasoline, and can freeze in the supply lines at cold temperatures. The isopropanol does not remove the water from the gasoline; rather, the isopropanol solubilizes the water in the gasoline. Once [[soluble]], the water does not pose the same risk as insoluble water as it will no longer accumulate in the supply lines and freeze. Isopropanol is often sold in aerosol cans as a [[windshield|windscreen]] de-icer. | ||
| − | Isopropanol is used as a water-drying aid for treating [[otitis externa]], better known as swimmer's ear.<ref>http://www.mcw.edu/pediatricoto/CommonHealthProblems/OtitisExternaSwimmersEar.htm</ref> | + | Isopropanol is used as a water-drying aid for treating [[otitis externa]], better known as swimmer's ear.<ref>{{citation | url = http://www.mcw.edu/pediatricoto/CommonHealthProblems/OtitisExternaSwimmersEar.htm}}.</ref> |
==Chemistry== | ==Chemistry== | ||
| − | Unlike [[ethanol]] or [[methanol]], isopropanol can be separated from aqueous solutions by adding a salt such as [[sodium chloride]], [[sodium sulfate]], or any of several other inorganic salts, since the alcohol is much less soluble in saline solutions than in salt-free water <ref> | + | Unlike [[ethanol]] or [[methanol]], isopropanol can be separated from aqueous solutions by adding a salt such as [[sodium chloride]], [[sodium sulfate]], or any of several other inorganic salts, since the alcohol is much less soluble in saline solutions than in salt-free water <ref>{{Merck9th|5069}}.</ref> The process is colloquially called [[salting out]], and causes concentrated isopropanol to separate into a distinct layer. |
Being a secondary alcohol, isopropanol can be [[Organic redox reaction|oxidized]] to the corresponding [[ketone]] [[acetone]]. This can be achieved using oxidizing agents such as [[chromic acid]], or by [[dehydrogenation]] of isopropanol over a heated copper [[catalyst]]: | Being a secondary alcohol, isopropanol can be [[Organic redox reaction|oxidized]] to the corresponding [[ketone]] [[acetone]]. This can be achieved using oxidizing agents such as [[chromic acid]], or by [[dehydrogenation]] of isopropanol over a heated copper [[catalyst]]: | ||
| − | :(CH<sub>3</sub>)<sub>2</sub>CH-OH → | + | :(CH<sub>3</sub>)<sub>2</sub>CH-OH → (CH<sub>3</sub>)<sub>2</sub>CO + H<sub>2</sub> |
| − | |||
| − | |||
| − | Isopropanol | + | Isopropanol may be converted to [[2-bromopropane]] using [[phosphorus tribromide]], or dehydrated to [[propylene]] by heating with [[sulfuric acid]]. |
| − | + | Isopropanol is often used as a [[hydride]] source in the [[Meerwein–Ponndorf–Verley reduction]]. | |
| − | + | Like most alcohols, isopropanol reacts with active [[metal]]s such as [[potassium]] to form [[alkoxide]]s which can be called ''isopropoxides''. The reaction with [[aluminium]] (initiated by a trace of [[mercury]]) is used to prepare the catalyst [[aluminium isopropoxide]]. | |
==Safety== | ==Safety== | ||
| − | Isopropyl alcohol vapor is more dense than air and is highly [[flammable]] with a very wide combustible range. It should be kept away from heat and open flame. When mixed with air or other oxidizers it can explode through [[deflagration]].<ref name = | + | Isopropyl alcohol vapor is more dense than air and is highly [[flammable]] with a very wide combustible range. It should be kept away from heat and open flame. When mixed with air or other oxidizers it can explode through [[deflagration]].<ref name="OxfordMSDS">{{citation | url = http://physchem.ox.ac.uk/MSDS/PR/2-propanol.html | publisher = Department of Chemistry, University of Oxford | webpage = Safety (MSDS) data for 2-propanol | accessdate=2006-09-28}}</ref> Isopropyl alcohol has also been reported to form explosive [[organic peroxide|peroxides]].<ref name="OxfordMSDS"/><ref>{{citation | publisher = Brookhaven National Laboratory | webpage = |
| − | Chemical Safety Hazard Alert | + | Chemical Safety Hazard Alert – Isopropanol, Peroxides Result in Explosion & Injury | last = Hess | first = R. K. | date = June 1997 | url = http://www.bnl.gov/esh/shsd/programs/Program_Area_Chemicals_Hazard_Alert_Isopropanol.asp}}</ref> |
| − | Like many organic solvents, long term application to the skin can cause [[defatting]] | + | Like many organic solvents, long term application to the skin can cause [[defatting]].<ref name="ICSC"/> |
==Toxicology== | ==Toxicology== | ||
| − | + | Isopropanol is oxidized by the liver into [[acetone]] by [[alcohol dehydrogenase]]. [[Symptom]]s of isopropanol poisoning include [[flushing (physiology)|flushing]], [[headache]], [[dizziness]], [[CNS depression]], [[nausea]], [[vomiting]], [[anesthesia]], and [[coma]]. Use in well-ventilated areas and use protective gloves while using. Poisoning can occur from ingestion, inhalation, or absorption. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | Isopropanol is about twice as toxic as [[ethanol]], and acts as a [[central nervous system|central nervous system (CNS)]] [[depressant]]. Its metabolite, [[acetone]], is a CNS depressant in its own right. Around 15 g of isopropanol can have a toxic effect on a 70 kg human if left untreated. However, it is not nearly as toxic as methanol or [[ethylene glycol]]. Isopropyl alcohol does not cause an [[anion gap acidosis]] (in which as lowered blood serum pH causes depletion of [[bicarbonate]] anion) as do [[ethanol]] and [[methanol]]. Isopropanol does, however, produce an [[osmolal gap]] between the calculated and measured osmolalities of serum, as do the other alcohols. Overdoses may cause a fruity odor on the breath as a result of its metabolism to [[acetone]], which is not further metabolized.<ref>{{citation | last = Tiess | first = D. | journal = Z. Ges. Hygiene | volume = 31 | pages = 530–31 | year = 1985}}.</ref> | |
==References== | ==References== | ||
| Line 101: | Line 101: | ||
==External links== | ==External links== | ||
| − | * [http://www.osha.gov/SLTC/healthguidelines/isopropylalcohol/recognition.html | + | *{{ICSC|0554}} |
| − | + | *{{PGCH|0359}} | |
| + | * [http://www.osha.gov/SLTC/healthguidelines/isopropylalcohol/recognition.html U.S. OSHA guidelines for isopropyl alcohol] | ||
* [http://ddbonline.ddbst.de/AntoineCalculation/AntoineCalculationCGI.exe?component=2-Propanol Vapor pressure ] and [http://ddbonline.ddbst.de/DIPPR105DensityCalculation/DIPPR105CalculationCGI.exe?component=2-Propanol liquid density] calculation | * [http://ddbonline.ddbst.de/AntoineCalculation/AntoineCalculationCGI.exe?component=2-Propanol Vapor pressure ] and [http://ddbonline.ddbst.de/DIPPR105DensityCalculation/DIPPR105CalculationCGI.exe?component=2-Propanol liquid density] calculation | ||
* [http://www.labmanager.com/stips.asp?ID=41 Lab Manager Article on Working with Isopropyl Alcohol] | * [http://www.labmanager.com/stips.asp?ID=41 Lab Manager Article on Working with Isopropyl Alcohol] | ||
| − | |||
{{Antiseptics and disinfectants}} | {{Antiseptics and disinfectants}} | ||
Latest revision as of 11:34, 1 September 2009
| Isopropanol | |
|---|---|
| IUPAC name | propan-2-ol |
| Other names | 2-Propanol Isopropyl alcohol |
| Identifiers | |
| InChI | InChI=1/C3H8O/c1-3(2)4/h3-4H,1-2H3 |
| InChIKey | KFZMGEQAYNKOFK-UHFFFAOYAH |
| Standard InChI | InChI=1S/C3H8O/c1-3(2)4/h3-4H,1-2H3 |
| Standard InChIKey | KFZMGEQAYNKOFK-UHFFFAOYSA-N |
| CAS number | [] |
| EC number | |
| RTECS | NT8050000 |
| ChemSpider | |
| SMILES | |
| Properties[1][2] | |
| Chemical formula | C3H7OH |
| Molar mass | 60.095 g/mol |
| Appearance | colorless liquid |
| Density | 0.786 g/cm3 (20 ºC), liquid |
| Melting point |
−90 °C, 183 K, -130 °F |
| Boiling point |
82.3 °C, 355 K, 180 °F |
| Solubility in water | miscible |
| log P | 0.05 |
| Vapor pressure | 4.4 kPa (20 ºC) |
| Acidity (pKa) | 16.5 |
| λmax | 204 nm |
| Viscosity | 1.96 cP (25 °C) |
| Dipole moment | 1.66 D (gas) |
| Hazards[1][3][4] | |
| Material safety data sheet (MSDS) | ICSC |
| EU index number | 603-117-00-0 |
| GHS pictograms |  
|
| GHS signal word | DANGER |
| GHS hazard statements | H225, H319, H336 |
| Flash point | 12 °C |
| Autoignition temp. | 456 ºC |
| Explosive limits | 2–12% |
| PEL (U.S.) | 400 ppm TWA |
| Related compounds | |
| Other alcohols | Ethanol Propan-1-ol Butan-2-ol |
| Other compounds | Acetone |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Isopropanol, also known as isopropyl alcohol, propan-2-ol, 2-propanol, iso, isopro, isoprop, rubbing alcohol, or the abbreviation IPA, is a colorless, flammable chemical compound with a strong odor. It has the molecular formula C3H7OH and is the simplest example of a secondary alcohol, where the alcohol carbon is attached to two other carbons. It is an isomer of propanol.
Production
Isopropanol is produced by combining water and propene.[5] There are two processes for achieving this: indirect hydration via the sulfuric acid process and direct hydration. The former process, which can use low-quality propylene, predominates in the USA while the latter process, which requires high-purity propylene, is more commonly used in Europe. These processes give predominantly isopropyl alcohol rather than propan-1-ol because the addition of water or sulfuric acid to propylene follows Markovnikov's rule.
The indirect process reacts propylene with sulfuric acid to form a mixture of sulfate esters. Subsequent hydrolysis of these esters produces isopropanol. Direct hydration reacts propylene and water, either in gas or liquid phases, at high pressures in the presence of solid or supported acidic catalysts. Both processes require that the isopropanol be separated from water and other by-products by distillation. Isopropanol and water form an azeotrope and simple distillation gives a material which is 87.9% by weight isopropyl alcohol and 12.1% by weight water.[6] Pure (anhydrous) isopropanol is made by azeotropic distillation of the "wet" isopropyl alcohol using either diisopropyl ether or cyclohexane as azeotroping agents.
Uses
Isopropanol is cheaply available. Like acetone, it dissolves a wide range of nonpolar compounds. It is also relatively nontoxic and dries (evaporates) quickly. Thus it is used widely as a solvent and as a cleaning fluid, especially for dissolving lipophilic contaminants such as oil. Examples of this use include cleaning electronic devices such as contact pins (like those on ROM cartridges), magnetic tape and disk heads (such as those in audio and video tape recorders and floppy disk drives), the lenses of lasers in optical disc drives (e.g. CD, DVD) and removing thermal paste from IC packages (such as CPUs.) It is also used to clean LCD and glass computer monitor screens (at some risk to the anti-reflection coating of some screens), and used by many music shops to give second-hand or worn records newer-looking sheens (though it may leach plasticizer from vinyl, making it more rigid). Pure isopropanol should not be used to clean vinyl records. A basic reciepe for cleaning vinyl records is: for 1 litre, 50% isopropanol, 50% demineralised water, one or two drops of pure detergent such as Kodak PhotoFlo. It cleans white boards and other unwanted ink related marks very well (at the risk of damaging the non-stick surface of the white board). Isopropanol also works well at removing smudges, dirt, and fingerprints from cell phones and PDAs. It is effective at removing residual glue from some sticky labels (but some other adhesives used on tapes and paper labels are resistant to it.) It can also be used to remove stains from most fabrics, wood, cotton, etc. Isopropanol is also used to remove brake fluid traces from hydraulic disk brake systems, so that the brake fluid (usually DOT 3, DOT 4 or mineral oil) does not contaminate the brake pads, which would result in poor braking. In addition it can also be used to clean paintballs or other oil based products so that they may be reused, commonly known as "repainting".
As a preservative (for biological specimens) isopropanol provides a cost-effective (when compared to pure ethanol) and comparatively non-toxic alternative to formaldehyde and other synthetic preservatives. When used for the preservation of specimens in solution concentrations of 90–99% are optimal, though concentrations as low as 70% can be used in emergencies.
Sterilizing pads typically contain a 60–70% solution of isopropanol in water. Isopropanol is also commonly used as a cleaner and solvent in industry.
Isopropanol is a major ingredient in "gas dryer" fuel additives. In significant quantities, water is a problem in fuel tanks, as it separates from the gasoline, and can freeze in the supply lines at cold temperatures. The isopropanol does not remove the water from the gasoline; rather, the isopropanol solubilizes the water in the gasoline. Once soluble, the water does not pose the same risk as insoluble water as it will no longer accumulate in the supply lines and freeze. Isopropanol is often sold in aerosol cans as a windscreen de-icer.
Isopropanol is used as a water-drying aid for treating otitis externa, better known as swimmer's ear.[7]
Chemistry
Unlike ethanol or methanol, isopropanol can be separated from aqueous solutions by adding a salt such as sodium chloride, sodium sulfate, or any of several other inorganic salts, since the alcohol is much less soluble in saline solutions than in salt-free water [8] The process is colloquially called salting out, and causes concentrated isopropanol to separate into a distinct layer.
Being a secondary alcohol, isopropanol can be oxidized to the corresponding ketone acetone. This can be achieved using oxidizing agents such as chromic acid, or by dehydrogenation of isopropanol over a heated copper catalyst:
- (CH3)2CH-OH → (CH3)2CO + H2
Isopropanol may be converted to 2-bromopropane using phosphorus tribromide, or dehydrated to propylene by heating with sulfuric acid.
Isopropanol is often used as a hydride source in the Meerwein–Ponndorf–Verley reduction.
Like most alcohols, isopropanol reacts with active metals such as potassium to form alkoxides which can be called isopropoxides. The reaction with aluminium (initiated by a trace of mercury) is used to prepare the catalyst aluminium isopropoxide.
Safety
Isopropyl alcohol vapor is more dense than air and is highly flammable with a very wide combustible range. It should be kept away from heat and open flame. When mixed with air or other oxidizers it can explode through deflagration.[9] Isopropyl alcohol has also been reported to form explosive peroxides.[9][10]
Like many organic solvents, long term application to the skin can cause defatting.[1]
Toxicology
Isopropanol is oxidized by the liver into acetone by alcohol dehydrogenase. Symptoms of isopropanol poisoning include flushing, headache, dizziness, CNS depression, nausea, vomiting, anesthesia, and coma. Use in well-ventilated areas and use protective gloves while using. Poisoning can occur from ingestion, inhalation, or absorption.
Isopropanol is about twice as toxic as ethanol, and acts as a central nervous system (CNS) depressant. Its metabolite, acetone, is a CNS depressant in its own right. Around 15 g of isopropanol can have a toxic effect on a 70 kg human if left untreated. However, it is not nearly as toxic as methanol or ethylene glycol. Isopropyl alcohol does not cause an anion gap acidosis (in which as lowered blood serum pH causes depletion of bicarbonate anion) as do ethanol and methanol. Isopropanol does, however, produce an osmolal gap between the calculated and measured osmolalities of serum, as do the other alcohols. Overdoses may cause a fruity odor on the breath as a result of its metabolism to acetone, which is not further metabolized.[11]
References
- ↑ 1.0 1.1 1.2 Isopropyl alcohol; International Chemical Safety Card 0554; International Labour Organization: Geneva, March 1999, <http://www.inchem.org/documents/icsc/icsc/eics0554.htm>.
- ↑ Yaws, Carl L. Chemical Properties Handbook: Physical, Thermodynamic, Environmental, Transport, Safety, and Health Related Properties for Organic and Inorganic Chemicals; McGraw-Hill: New York, 1998. ISBN 0070734011.
- ↑ Index no. 603-117-00-0 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 493.
- ↑ Isopropyl alcohol. In Pocket Guide to Chemical Hazards; U.S. Department of Health and Human Services (NIOSH) Publication No. 2005-149; Government Printing Office: Washington, DC, 2005. ISBN 9780160727511, <http://www.cdc.gov/niosh/npg/default.html>.
- ↑ Logsdon, John E.; Loke, Richard A. Isopropyl Alcohol. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley: New York, 2004.
- ↑ Handbook of Chemistry and Physics, 44th ed.; Chemical Rubber Co.: Cleveland, OH, 1963; pp 2143–84.
- ↑ , <http://www.mcw.edu/pediatricoto/CommonHealthProblems/OtitisExternaSwimmersEar.htm>.
- ↑ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 9th ed.; Merck, 5069.
- ↑ 9.0 9.1 Safety (MSDS) data for 2-propanol, <http://physchem.ox.ac.uk/MSDS/PR/2-propanol.html> (accessed 28 September 2006), Department of Chemistry, University of Oxford
- ↑ Hess, R. K. Chemical Safety Hazard Alert – Isopropanol, Peroxides Result in Explosion & Injury, <http://www.bnl.gov/esh/shsd/programs/Program_Area_Chemicals_Hazard_Alert_Isopropanol.asp>, Brookhaven National Laboratory, June 1997
- ↑ Tiess, D. Z. Ges. Hygiene 1985, 31, 530–31.
External links
- International Chemical Safety Card 0554
- NIOSH Pocket Guide to Chemical Hazards 0359
- U.S. OSHA guidelines for isopropyl alcohol
- Vapor pressure and liquid density calculation
- Lab Manager Article on Working with Isopropyl Alcohol
Template:Antiseptics and disinfectants
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Isopropyl alcohol". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |