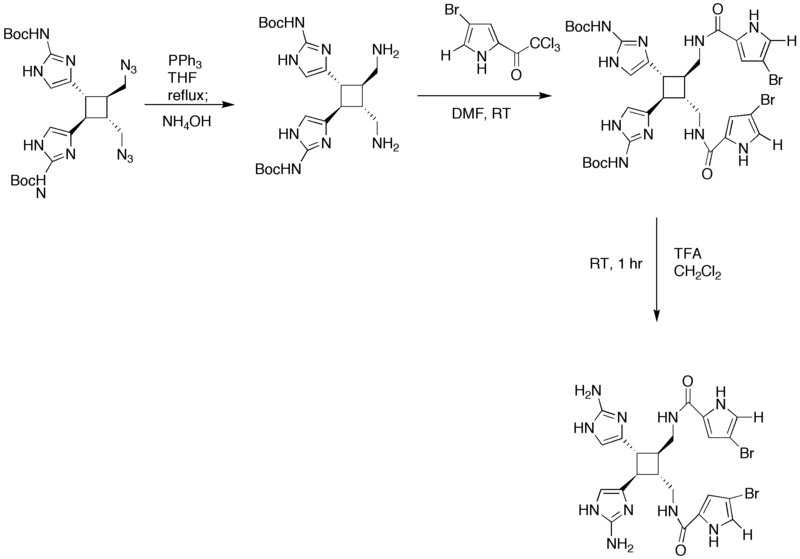Difference between revisions of "Birman's synthesis of sceptrin"
Fayettmr190 (talk | contribs) (changed retro diagram) |
Physchim62 (talk | contribs) |
||
| (11 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | Sceptrin is a useful naturally occurring product found in ''Agelas sceptrum'', a sponge that inhabits the Glover Reef in Belize. The chemical has anti-bacterial, antiviral, and anti-histaminic properties. Sceptrin also has anti-muscarinic properties. The term muscarinic is derived from [[muscarine]], an alkaloid poison found in certain fungi. | |
| − | |||
| − | |||
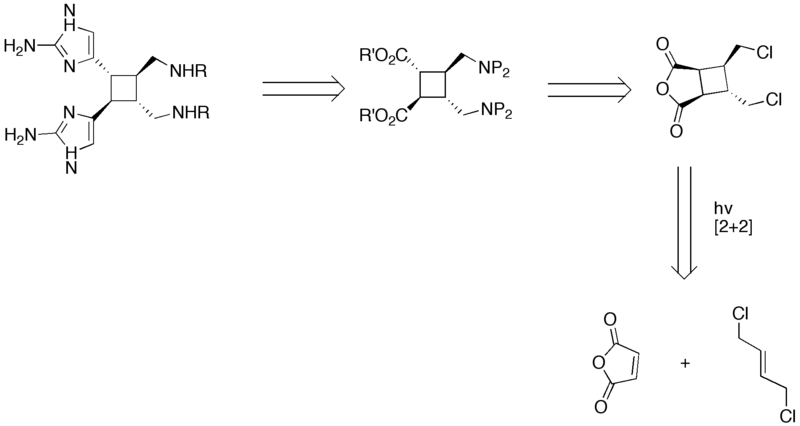
== Retrosynthesis == | == Retrosynthesis == | ||
| − | + | [[Image:Synthesis_of_Sceptrin_IV.png|800px|Retrosynthetic analysis of Sceptrin]] | |
| − | [[Image:Synthesis_of_Sceptrin_IV. | ||
| − | |||
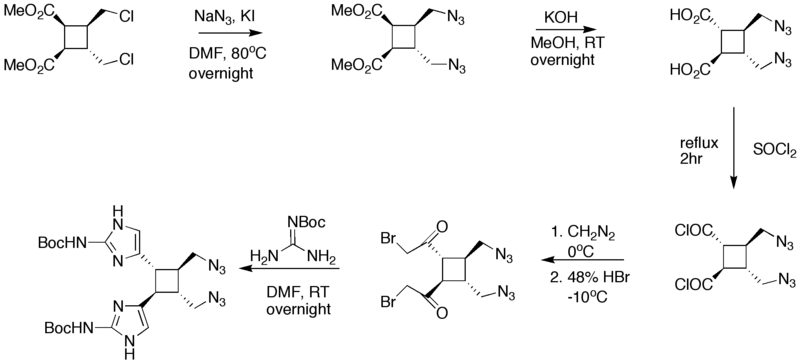
== Synthesis of Starting compound == | == Synthesis of Starting compound == | ||
| − | [[Image:]] | + | [[Image:Synthesis_of_Sceptrin_III.png|800px|Synthesis of Sceptrin starting material]] |
| − | |||
== Synthesis of Sceptrin (2nd Generation) == | == Synthesis of Sceptrin (2nd Generation) == | ||
| − | [[Image: | + | [[Image:Synthesis_of_Sceptrin.png|800px|Total synthesis of Sceptrin, part 1]] |
| − | |||
| − | |||
| − | |||
| + | [[Image:Synthesis_of_Sceptrin_II.png|800px|Total synthesis of Sceptrin, part 2]] | ||
== Inside the synthesis == | == Inside the synthesis == | ||
| − | + | The starting four member ring is made via a [2+2] addition in the presence of UV light. In step 3 of the synthesis, the KOH/MeOH changed the orientation of carbon atom in the first position from cis to trans. Guanidine was added in DMF to convert the bromoacetyl groups into [[imidazole]] rings. The addition of PPh<sub>3</sub> in NH<sub>3</sub> fosters the change of the azides into amines. | |
== Conclusions == | == Conclusions == | ||
| − | + | It should also be noted that the compound is stereoselective. An interesting feature of this compound lies in the fact that the central ring is a [[cyclobutane]] ring, which in usual circumstances is not very stable due to ring strain. The overall synthesis of sceptrin is convergent. | |
| − | |||
| − | |||
==References== | ==References== | ||
| − | # Birman | + | # {{citation | last1 = Birman | first1 = Vladimir B. | last2 = Jiang | first2 = Xun-Tian | title = Synthesis of Sceptrin Alkaloids | journal = Org. Lett. | year = 2004 | volume = 6 | issue = 14 | pages = 2369–71 | doi=10.1021/ol049283g}}. |
| − | # Baran | + | # {{citation | last1 = Baran | first1 = Phil S. | last2 = Zografos | first2 = Alexandros L. | last3 = O'Malley | first3 = Daniel P. | title = Short Total Synthesis of (±)-Sceptrin | journal = J. Am. Chem. Soc. | year = 2004 | volume = 126 | issue = 12 | pages = 3726–27 | doi=10.1021/ja049648s}}. |
| − | #Walker | + | # {{citation | last1 = Walker | first1 = Roger P. | last2 = Faulkner | first2 = D. John | first3 = Donna | last3 = Van Engen | first4 = Jon | last4 = Clardy | title = Sceptrin, an antimicrobial agent from the sponge ''Agelas sceptrum'' | journal = J. Am. Chem. Soc. | year = 1981 | volume = 103 | page = 6772–73 | doi = 10.1021/ja00412a052}}. |
[[Category:Chemistry 444 pages]] | [[Category:Chemistry 444 pages]] | ||
[[Category:Total syntheses]] | [[Category:Total syntheses]] | ||
Latest revision as of 22:12, 17 August 2009
Sceptrin is a useful naturally occurring product found in Agelas sceptrum, a sponge that inhabits the Glover Reef in Belize. The chemical has anti-bacterial, antiviral, and anti-histaminic properties. Sceptrin also has anti-muscarinic properties. The term muscarinic is derived from muscarine, an alkaloid poison found in certain fungi.
Contents
Retrosynthesis
Synthesis of Starting compound
Synthesis of Sceptrin (2nd Generation)
Inside the synthesis
The starting four member ring is made via a [2+2] addition in the presence of UV light. In step 3 of the synthesis, the KOH/MeOH changed the orientation of carbon atom in the first position from cis to trans. Guanidine was added in DMF to convert the bromoacetyl groups into imidazole rings. The addition of PPh3 in NH3 fosters the change of the azides into amines.
Conclusions
It should also be noted that the compound is stereoselective. An interesting feature of this compound lies in the fact that the central ring is a cyclobutane ring, which in usual circumstances is not very stable due to ring strain. The overall synthesis of sceptrin is convergent.
References
- Birman, Vladimir B.; Jiang, Xun-Tian Synthesis of Sceptrin Alkaloids. Org. Lett. 2004, 6 (14), 2369–71. DOI: 10.1021/ol049283g.
- Baran, Phil S.; Zografos, Alexandros L.; O'Malley, Daniel P. Short Total Synthesis of (±)-Sceptrin. J. Am. Chem. Soc. 2004, 126 (12), 3726–27. DOI: 10.1021/ja049648s.
- Walker, Roger P.; Faulkner, D. John; Van Engen, Donna; Clardy, Jon Sceptrin, an antimicrobial agent from the sponge Agelas sceptrum. J. Am. Chem. Soc. 1981, 103, 6772–73. DOI: 10.1021/ja00412a052.