Difference between revisions of "Sulfurous acid"
Physchim62 (talk | contribs) (Imported from http://en.wikipedia.org/w/index.php?title=Sulfurous_acid&oldid=303432116) |
Physchim62 (talk | contribs) (cleanup) |
||
| Line 9: | Line 9: | ||
| IUPACName = Sulfurous acid | | IUPACName = Sulfurous acid | ||
| Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| + | | InChI = 1/H2O3S/c1-4(2)3/h(H2,1,2,3) | ||
| + | | StdInChI = 1S/H2O3S/c1-4(2)3/h(H2,1,2,3) | ||
| + | | InChIKey = LSNNMFCWUKXFEE-UHFFFAOYAJ | ||
| + | | StdInChIKey = LSNNMFCWUKXFEE-UHFFFAOYSA-N | ||
| CASNo = 7782-99-2 | | CASNo = 7782-99-2 | ||
| CASNo_Ref = {{cascite}} | | CASNo_Ref = {{cascite}} | ||
| + | | EC-number = 231-973-1 | ||
| + | | ChemSpiderID = 1069 | ||
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| Line 16: | Line 22: | ||
| MolarMass = 82.07 g/mol | | MolarMass = 82.07 g/mol | ||
}} | }} | ||
| − | | Section7 = {{Chembox Hazards | + | | Section7 = {{Chembox Hazards <!-- Hazards as for sulfur dioxide (except that it isn't {{GHS04|Press. Gas}} ) --> |
| − | | ExternalMSDS = | + | | Reference = <ref>{{CLP Regulation|index=016-011-00-9|page=399}}</ref> |
| + | | ExternalMSDS = {{ICSC-short|00|74}} | ||
| EUIndex = 016-011-00-9 | | EUIndex = 016-011-00-9 | ||
| − | | | + | | GHSPictograms = {{GHS06|Acute Tox. (inhal.) 3}}{{GHS05|Skin Corr. 1B}} |
| − | + | | GHSSignalWord = DANGER | |
| − | | | + | | HPhrases = {{H-phrases|331|314}} |
| FlashPt = Non-flammable | | FlashPt = Non-flammable | ||
}} | }} | ||
| Line 29: | Line 36: | ||
}} | }} | ||
| − | '''Sulfurous acid''' is the [[chemical compound]] with the [[chemical formula|formula]] H<sub>2</sub>SO<sub>3</sub>. There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase.<ref>{{ | + | '''Sulfurous acid''' is the [[chemical compound]] with the [[chemical formula|formula]] H<sub>2</sub>SO<sub>3</sub>. There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase.<ref>{{citation | first1 = D. | last1 = Sülzle | first2 = M. | last2 = Verhoeven | first3 = J. K. | last3 = Terlouw | first4 = H. | last4 = Schwarz | title = Generation and Characterization of Sulfurous Acid (H<sub>2</sub>SO<sub>3</sub>) and of Its Radical Cation as Stable Species in the Gas Phase | journal = Angew. Chem., Int. Ed. Engl. | volume = 27 | pages = 1533–34 | year = 1988 | doi = 10.1002/anie.198815331}}.</ref> The conjugate bases of this elusive acid are, however, common anions, [[bisulfite]] (or hydrogensulfite) and [[sulfite]]. |
| − | [[Raman spectroscopy|Raman spectra]] of solutions of [[sulfur dioxide]] in water show only signals due to the SO<sub>2</sub> molecule and the bisulfite ion, HSO<sub>3</sub><sup>−</sup>.<ref>{{Jolly2nd}}</ref> The intensities of the signals are consistent with the following [[chemical equilibrium|equilibrium]]: | + | [[Raman spectroscopy|Raman spectra]] of solutions of [[sulfur dioxide]] in water show only signals due to the SO<sub>2</sub> molecule and the bisulfite ion, HSO<sub>3</sub><sup>−</sup>.<ref>{{Jolly2nd|page=227}}.</ref> The intensities of the signals are consistent with the following [[chemical equilibrium|equilibrium]]: |
::SO<sub>2</sub> + H<sub>2</sub>O {{eqm}} HSO<sub>3</sub><sup>−</sup> + H<sup>+</sup> | ::SO<sub>2</sub> + H<sub>2</sub>O {{eqm}} HSO<sub>3</sub><sup>−</sup> + H<sup>+</sup> | ||
| Line 39: | Line 46: | ||
==References== | ==References== | ||
| − | + | {{reflist}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Sulfites|*]] | [[Category:Sulfites|*]] | ||
Revision as of 20:09, 23 August 2009
| Sulfurous acid | |
|---|---|
| |

| |
| IUPAC name | Sulfurous acid |
| Identifiers | |
| InChI | InChI=1/H2O3S/c1-4(2)3/h(H2,1,2,3) |
| InChIKey | LSNNMFCWUKXFEE-UHFFFAOYAJ |
| Standard InChI | InChI=1S/H2O3S/c1-4(2)3/h(H2,1,2,3) |
| Standard InChIKey | LSNNMFCWUKXFEE-UHFFFAOYSA-N |
| CAS number | [] |
| EC number | |
| ChemSpider | |
| Properties | |
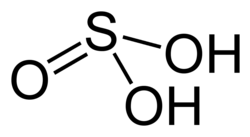
| Chemical formula | H2SO3 |
| Molar mass | 82.07 g/mol |
| Hazards[1] | |
| Material safety data sheet (MSDS) | ICSC |
| EU index number | 016-011-00-9 |
| GHS pictograms |  
|
| GHS signal word | DANGER |
| GHS hazard statements | H331, H314 |
| Flash point | Non-flammable |
| Related compounds | |
| Other compounds | Sulfur dioxide Sulfuric acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Sulfurous acid is the chemical compound with the formula H2SO3. There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase.[2] The conjugate bases of this elusive acid are, however, common anions, bisulfite (or hydrogensulfite) and sulfite.
Raman spectra of solutions of sulfur dioxide in water show only signals due to the SO2 molecule and the bisulfite ion, HSO3−.[3] The intensities of the signals are consistent with the following equilibrium:
- SO2 + H2O ⇌ HSO3− + H+
- Ka = 1.54 × 10−2; pKa = 1.81.
- SO2 + H2O ⇌ HSO3− + H+
Aqueous solutions of sulfur dioxide, which sometimes are referred to as sulfurous acid are used as reducing agents and as disinfectants, as are solutions of bisulfite and sulfite salts. They are also mild bleaches, and are used for materials which may be damaged by chlorine-containing bleaches.
References
- ↑ Index no. 016-011-00-9 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 399.
- ↑ Sülzle, D.; Verhoeven, M.; Terlouw, J. K.; Schwarz, H. Generation and Characterization of Sulfurous Acid (H2SO3) and of Its Radical Cation as Stable Species in the Gas Phase. Angew. Chem., Int. Ed. Engl. 1988, 27, 1533–34. DOI: 10.1002/anie.198815331.
- ↑ Jolly, William L. Modern Inorganic Chemistry, 2nd ed.; McGraw-Hill: New York, 1991; p 227. ISBN 0-07-032768-8.
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Sulfurous acid". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |