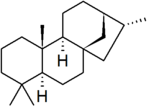Difference between revisions of "Kaurane"
Physchim62 (talk | contribs) |
Physchim62 (talk | contribs) |
||
| Line 10: | Line 10: | ||
'''Kaurane''' is a [[parent hydride]] used in the [[IUPAC nomenclature of natural products]] and also, distinctly, in [[CAS nomenclature]]. It is a [[diterpene]] with a rigid four-ring structure and six [[Chirality|chiral centres]]. | '''Kaurane''' is a [[parent hydride]] used in the [[IUPAC nomenclature of natural products]] and also, distinctly, in [[CAS nomenclature]]. It is a [[diterpene]] with a rigid four-ring structure and six [[Chirality|chiral centres]]. | ||
| + | [[File:Kaurane numbered.png|thumb|right|The conventional numbering of kaurane: for the numbering of the methyl groups and C-18 and C-19, see text.]] | ||
The stereochemistry of the six chiral centres is defined by convention: however, IUPAC and CAS use different conventional stereochemistry, which also leads to a slight difference in numbering between the two systems. IUPAC conventional kaurane has (5''S'',8''S'',9''S'',10''S'',13''S'',15''R'')-stereochemistry, and is drawn with the five-membered ring receding into the plane of the image.<ref name="IUPAC">{{IUPAC natural products 1999}}.</ref> CAS conventional kaurane has (5''S'',8''R'',9''S'',10''S'',13''R'',15''R'')-stereochemistry, and is drawn with the five-membered ring protruding from the plane of the image.<ref name="CAS">{{citation | contribution = 57817-89-7 – Kaur-16-en-18-oic acid, 13-[(2-''O''-β-<small>D</small>-glucopyranosyl-β-<small>D</small>-glucopyranosyl)oxy]-, β-<small>D</small>-glucopyranosyl ester, (4α)- | url = http://www.commonchemistry.org/ChemicalDetail.aspx?ref=57817-89-7 | title = Common Chemistry | publisher = Chemical Abstracts Service | accessdate = 2009-09-05}}.</ref> Carbon-19 (one of the two methyl groups attached to carbon-4) is on the same side of the molecule as the five-membered ring in both systems: hence it is receding into the plane of the image in IUPAC nomenclature and protruding from the plane of the image in CAS nomenclature. | The stereochemistry of the six chiral centres is defined by convention: however, IUPAC and CAS use different conventional stereochemistry, which also leads to a slight difference in numbering between the two systems. IUPAC conventional kaurane has (5''S'',8''S'',9''S'',10''S'',13''S'',15''R'')-stereochemistry, and is drawn with the five-membered ring receding into the plane of the image.<ref name="IUPAC">{{IUPAC natural products 1999}}.</ref> CAS conventional kaurane has (5''S'',8''R'',9''S'',10''S'',13''R'',15''R'')-stereochemistry, and is drawn with the five-membered ring protruding from the plane of the image.<ref name="CAS">{{citation | contribution = 57817-89-7 – Kaur-16-en-18-oic acid, 13-[(2-''O''-β-<small>D</small>-glucopyranosyl-β-<small>D</small>-glucopyranosyl)oxy]-, β-<small>D</small>-glucopyranosyl ester, (4α)- | url = http://www.commonchemistry.org/ChemicalDetail.aspx?ref=57817-89-7 | title = Common Chemistry | publisher = Chemical Abstracts Service | accessdate = 2009-09-05}}.</ref> Carbon-19 (one of the two methyl groups attached to carbon-4) is on the same side of the molecule as the five-membered ring in both systems: hence it is receding into the plane of the image in IUPAC nomenclature and protruding from the plane of the image in CAS nomenclature. | ||
Revision as of 16:53, 5 September 2009
Kaurane is a parent hydride used in the IUPAC nomenclature of natural products and also, distinctly, in CAS nomenclature. It is a diterpene with a rigid four-ring structure and six chiral centres.
The stereochemistry of the six chiral centres is defined by convention: however, IUPAC and CAS use different conventional stereochemistry, which also leads to a slight difference in numbering between the two systems. IUPAC conventional kaurane has (5S,8S,9S,10S,13S,15R)-stereochemistry, and is drawn with the five-membered ring receding into the plane of the image.[1] CAS conventional kaurane has (5S,8R,9S,10S,13R,15R)-stereochemistry, and is drawn with the five-membered ring protruding from the plane of the image.[2] Carbon-19 (one of the two methyl groups attached to carbon-4) is on the same side of the molecule as the five-membered ring in both systems: hence it is receding into the plane of the image in IUPAC nomenclature and protruding from the plane of the image in CAS nomenclature.
The name ent-kaurane is sometimes used to refer to the CAS conventional kaurane,[3] and so to distinguish it from the IUPAC stereochemistry, in particular by the International Union of Biochemistry and Molecular Biology (IUBMB).[4] It is also possible to explicitly specify the stereochemistry at carbons 8 and 13, giving:
- (8S,13S)-kaurane (IUPAC conventional)
- (8R,13R)-kaurane (CAS conventional)
Kaurane diterpenes have been extracted from a variety of plant species,[3][5] in particular Stevia sp., the source of the steviol glycosides stevioside and rebaudioside-A that have been used as artificial sweetners.[6] They are intermediates on the gibberellic acid pathway to several plant hormones known as gibberellins:[7] gibberellic acid is biosynthesized from ent-kaurene by six successive oxidations catalyzed by ent-kaurene oxidase (EC 1.14.13.78) and ent-kaurenic acid oxidase (EC 1.14.13.79).[4]
References
- ↑ Revised Section F: Natural Products and Related Compounds (IUPAC Recommendations 1999). Pure Appl. Chem., 71 (4), 587–643. DOI: 10.1351/pac199971040587.
- ↑ 57817-89-7 – Kaur-16-en-18-oic acid, 13-[(2-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy]-, β-D-glucopyranosyl ester, (4α)-. In Common Chemistry; Chemical Abstracts Service, <http://www.commonchemistry.org/ChemicalDetail.aspx?ref=57817-89-7>. (accessed 5 September 2009).
- ↑ 3.0 3.1 Nagashima, Fumihiro; Tanaka, Hironao; Takaoka, Shigeru; Asakawa, Yoshinori Ent-kaurane-type diterpenoids from the liverwort Jungermannia exsertifolia ssp. cordifolia. Phytochemistry 1996, 41 (4), 1129–41. DOI: 10.1016/0031-9422(95)00755-5. Arriaga-Giner, Francisco Javier; Rumbero, Angel; Wollenweber, Eckhard 16α,19-Diacetoxy-ent-kaurane, a New Natural Diterpene from the Exudate of Ozothamnus scutellifolius (Asteraceae). Z. Naturforsch. C 1999, 54, 602–4. Ding, Lan; Zhang, Zhang-Jing; Liu, Guo-An; Yang, Dong-Juan; Guo, Guo-Cong; Wang, Han; Sun, Kun Three New Cytotoxic ent-Kaurane Diterpenoids from Isodon weisiensis C. Y. Wu. Helv. Chim. Acta 2005, 88 (9), 2502–7. DOI: 10.1002/hlca.200590185. Batista, Ronan; García, Pablo A.; Castro, Maria A.; del Corral, José M. Miguel; San Feliciano, Arturo; de Oliveira, Alaíde B. New oxidized ent-kaurane and ent-norkaurane derivatives from kaurenoic acid. J. Braz. Chem. Soc. 2007, 18 (3), 622–27. DOI: 10.1590/S0103-50532007000300020. Zhao, Yong; Pu, Jian-Xin; Huang, Sheng-Xiong; Ding, Li-Sheng; Wu, Ying-Li; Li, Xian; Yang, Li-Bin; Xiao, Wei-Lie, et al. ent-Kaurane diterpenoids from Isodon pharicus. J. Nat. Prod. 2009, 72 (6), 988–93. PMID 19425589. DOI: 10.1021/np9000366.
- ↑ 4.0 4.1 EC 1.14.13.78 – ent-kaurene oxidase. In ExPASy Proteomics Server; Swiss Institute of Bioinformatics, <http://www.expasy.org/cgi-bin/nicezyme.pl?1.14.13.78>. (accessed 5 September 2009). EC 1.14.13.79 – ent-kaureneic acid oxidase. In ExPASy Proteomics Server; Swiss Institute of Bioinformatics, <http://www.expasy.org/cgi-bin/nicezyme.pl?1.14.13.79>. (accessed 5 September 2009).
- ↑ Nagashima, Fumihiro; Kondoh, Masuo; Uematsu, Toshinari; Nishiyama, Akiko; Saito, Sayaka; Sato, Masao; Asakawa, Yoshinori Cytotoxic and apoptosis-inducing ent-kaurane-type diterpenoids from the Japanese liverwort Jungermannia truncata nees. Chem. Pharm. Bull. 2002, 50 (6), 808–13. DOI: 10.1248/cpb.50.808. Bruno, Maurizio; Piozzi, Franco; Arnold, Nelly Apostolides; Başer, K. Hüsnü Can; Tabanca, Nurhayat; Kirimer, Neşe Kaurane Diterpenoids from Three Sideritis Species. Turk. J. Chem. 2005, 29 (1), 61–64, <http://journals.tubitak.gov.tr/chem/issues/kim-05-29-1/kim-29-1-7-0402-1.pdf>. Kim, Ki Hyun; Choi, Sang Un; Lee, Kang Ro Diterpene glycosides from the seeds of Pharbitis nil. J. Nat. Prod. 2009, 72 (6), 1121–27. PMID 19435339. DOI: 10.1021/np900101t.
- ↑ Jaitak, Vikas; Bikram Singh, Bandna; Kaul, V. K. An efficient microwave-assisted extraction process of stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni). Phytochem. Anal. 2009, 20 (3), 240–45. PMID 19358287. DOI: 10.1002/pca.1120.
- ↑ Brandle, J. E.; Telmer, P. G. Steviol glycoside biosynthesis. Phytochemistry 2007, 68 (14), 1855–63. DOI: 10.1016/j.phytochem.2007.02.010.
Further reading
- Lee, Jeong-Hyung; Koo, Tae Hyeon; Hwang, Bang Yeon; Lee, Jung Joon Kaurane Diterpene, Kamebakaurin, Inhibits NF-κB by Directly Targeting the DNA-binding Activity of p50 and Blocks the Expression of Antiapoptotic NF-κB Target Genes. J. Biol. Chem., 277 (21), 18411–20. DOI: 10.1074/jbc.M201368200.
External links
- Kaurane Chemical Structure (shows the IUPAC conventional structure)
- Diterpenes, Kaurane – Research News and Information
| Error creating thumbnail: Unable to save thumbnail to destination |
This page is currently licensed under the Creative Commons Attribution 3.0 Unported license and any later versions of that license. |