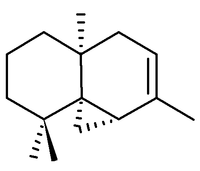
Thujopsene
Thujopsene
| Thujopsene | |
|---|---|
| |
| IUPAC name | (1aS,4aS,8aS)-2,4a,8,8-tetramethyl-1,1a,4,4a,5,6,7,8-octahydrocyclopropa[d]naphthalene |
| Other names | Widdrene |
| Identifiers | |
| InChI | InChI=1/C15H24/c1-11-6-9-14(4)8-5-7-13(2,3)15(14)10-12(11)15/h6,12H,5,7-10H2,1-4H3/t12-,14-,15-/m0/s1 |
| InChIKey | WXQGPFZDVCRBME-QEJZJMRPBP |
| CAS number | [] |
| ChemSpider | |
| PubChem | |
| SMILES | |
| Properties | |
| Chemical formula | C15H24 |
| Molar mass | 204.35 g/mol |
| Appearance | Clear, colourless liquid |
| Density | 0.936 g/mL (liquid at 20°C) |
| Boiling point |
258-260 °C, 271 K, -178 °F |
| Solubility in water | Very low |
| Specific rotation [α]D | −110° (CHCl3) |
| Hazards | |
| Main hazards | flammable |
| NFPA 704 | |
| Flash point | 104 °C |
| Related compounds | |
| Other alkene | Cedrene, Germacrene |
| Other compounds | Cedrol, Widdrol |
| Template:Tick(what is this?) (verify) Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Thujopsene is a tricyclic sesquiterpene commonly found in oils isolated from trees of the Cupressaceae (cypress) family, especially the geni Thujopsis, Juniperus (juniper) and Widdringtonia. It undergoes acetylation with rearrangement to produce a compound with a woody odour which is used for perfumery.
Structure
The correct structure and relative stereochemistry (cis) was first established in 1961 by T. Norin, [1] and confirmed by total synthesis in 1963.[2]. The absolute configuration was established by T. Norin in 1963,[3] confirming work on the related sesquiterpene widdrol by C. Enzell. [4]
Occurrence
Thujopsene is the principal component in several natural Cupressacea oils. It was first isolated from hiba oil by Yano (1913) and Uchida (1928)[5] .
In 1958, thujopsene was identified (initially as "widdrene") in the heartwood of the five Widdringtonia species by Erdtman and Thomas in 1958. [6]
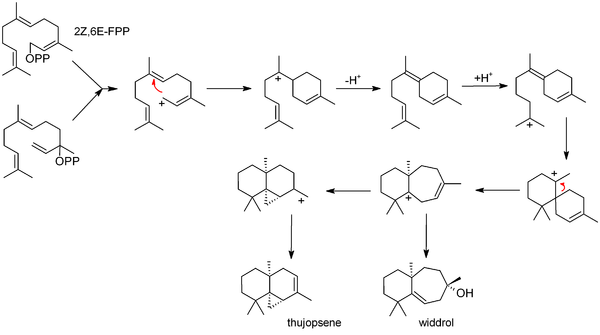
Biosynthesis
The commonly accepted biosynthesis[7] begins with 2Z,6E-farnesyl pyrophosphate (FPP) or its allylic isomer, nerolidyl pyrophosphate. These starting materials can both produce an allylic ion that cyclises to bisabolene. Bisabolene then (after reprotonation) cyclises further to form a seven-membered ring; this may simply hydrate (forming widdrol), or it may cyclize again, forming the three membered ring system of thujopsene.
Bisabolene is also invoked as an intermediate in the related biosyntheses of juvabione, cedrene and cuparene.
- ↑ Norin, Torbjörn (1961). "The Chemistry of the Natural Order Cupressales. 40. The Structure of Thujopsene and Hinokiic Acid." (in English). Acta Chemica Scandinavica 15: 1676–1694. doi:10.3891/acta.chem.scand.15-1676.
- ↑ Dauben, William G.; Arnold C. Ashcraft (1963). "The Total Synthesis of (±)-Thujopsene". Journal of the American Chemical Society 85: 3673–3676. doi:10.1021/ja00905a032.
- ↑ Norin, Torbjörn (1963). "The Chemistry of the Natural Order Cupressales. 49. The Configuration of Thujopsene." (in English). Acta Chemica Scandinavica 17: 738–748. doi:10.3891/acta.chem.scand.17-0738.
- ↑ Enzell, C. (1962). "The Chemistry of the Natural Order Cupressales. 47. The Structures and Absolute Configurations of Widdrol and Widdrol-alpha-epoxide." (in English). Acta Chemica Scandinavica 16: 1553–1568. doi:10.3891/acta.chem.scand.16-1553.
- ↑ Johnson, Carl R.; Michael R. Barbachyn (1982). "β-Hydroxysulfoximine-Directed Simmons-Smith Cyclopropanations. Synthesis of (-)- and (+)-Thujopsene". Journal of the American Chemical Society 104: 4290–4291. doi:10.1021/ja00379a060.
- ↑ Erdtman, H.; B. R. Thomas (1958). "The Chemistry of Natural Order Cupressales. XX. Heartwood Constituents of the Genus Widdringtonia." (in English). Acta Chemica Scandinavica 12: 267–273. doi:10.3891/acta.chem.scand.12-0267.
- ↑ (1994) Natural Products: their chemistry and biological significance (in English). Harlow, Essex, UK: Longman, 319-320. ISBN 0-582-06009-5.