Difference between revisions of "Ethanol"
Physchim62 (talk | contribs) (→Further reading) |
Physchim62 (talk | contribs) (→External links) |
||
| (11 intermediate revisions by the same user not shown) | |||
| Line 20: | Line 20: | ||
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| − | | Reference = <ref name="ICSC">{{ICSC-ref| | + | | Reference = <ref name="ICSC">{{ICSC-ref|0044|name=Ethanol (anhydrous)|date=October 2000}}.</ref><ref name="NIST">{{NIST chemistry|id=1S/C2H6O/c1-2-3/h3H,2H2,1H3|name=Ethyl alcohol|accessdate=2009-08-21}}.</ref> |
| C = 2|H = 6|O = 1 | | C = 2|H = 6|O = 1 | ||
| Appearance = colorless liquid | | Appearance = colorless liquid | ||
| Line 36: | Line 36: | ||
}} | }} | ||
| Section4 = {{Chembox Thermochemistry | | Section4 = {{Chembox Thermochemistry | ||
| − | | Reference = <ref name="NIST"/><ref>{{citation | last = Kelley | first = Kenneth K. | title = The heat capacities of ethyl and hexyl alcohols from 16°K to 298°K and the corresponding entropies and free energies | journal = J. Am. Chem. Soc. | year = 1929 | volume = 51 | issue = 3 | pages = 779–86 | doi = 10.1021/ja01378a016}}. {{citation | last = Green | first = J. H. S. | title = Revision of the values of the heats of formation of normal alcohols | journal = Chem. Ind. (London) | year = 1960 | pages = 1215–16}}. {{citation | last = Green | first = J. H. S. | title = Thermodynamic properties of organic oxygen compounds. Part 5. Ethyl alcohol | journal = Trans. Faraday Soc. | year = 1961 | volume = 57 | pages = 2132–37}}. {{citation | last1 = Chao | first1 = Jing | last2 = Rossini | first2 = F. D. | title = Heats of combustion, formation, and isomerization of nineteen alkanols | journal = J. Chem. Eng. Data | year = 1965 | volume = 10 | issue = 4 | pages = 374–79 | doi = 10.1021/je60027a022}}. {{citation | last1 = Haida | first1 = Osamu | last2 = Suga | first2 = Hiroshi | last3 = Seki | first3 = Syûzô | title = Calorimetric study of the glassy state. XII. Plural glass-transition phenomena of ethanol | journal = J. Chem. Thermodynam. | year = 1977 | volume = 9 | issue = 12 | pages = 1133–48 | doi = 10.1016/0021-9614(77)90115-X}}. {{citation | last1 = Majer | first1 = Vladimír | last2 = Svoboda | first2 = Václav | title = Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation | publisher = Blackwell | location = Oxford | year = 1985 | isbn = 0632015292}}.</ref> | + | | Reference = <ref name="NIST"/><ref>{{citation | last = Kelley | first = Kenneth K. | title = The heat capacities of ethyl and hexyl alcohols from 16°K to 298°K and the corresponding entropies and free energies | journal = J. Am. Chem. Soc. | year = 1929 | volume = 51 | issue = 3 | pages = 779–86 | doi = 10.1021/ja01378a016}}. {{citation | last = Green | first = J. H. S. | title = Revision of the values of the heats of formation of normal alcohols | journal = Chem. Ind. (London) | year = 1960 | pages = 1215–16}}. {{citation | last = Green | first = J. H. S. | title = Thermodynamic properties of organic oxygen compounds. Part 5. Ethyl alcohol | journal = Trans. Faraday Soc. | year = 1961 | volume = 57 | pages = 2132–37 | doi = 10.1039/TF9615702132}}. {{citation | last1 = Chao | first1 = Jing | last2 = Rossini | first2 = F. D. | title = Heats of combustion, formation, and isomerization of nineteen alkanols | journal = J. Chem. Eng. Data | year = 1965 | volume = 10 | issue = 4 | pages = 374–79 | doi = 10.1021/je60027a022}}. {{citation | last1 = Haida | first1 = Osamu | last2 = Suga | first2 = Hiroshi | last3 = Seki | first3 = Syûzô | title = Calorimetric study of the glassy state. XII. Plural glass-transition phenomena of ethanol | journal = J. Chem. Thermodynam. | year = 1977 | volume = 9 | issue = 12 | pages = 1133–48 | doi = 10.1016/0021-9614(77)90115-X}}. {{citation | last1 = Majer | first1 = Vladimír | last2 = Svoboda | first2 = Václav | title = Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation | publisher = Blackwell | location = Oxford | year = 1985 | isbn = 0632015292}}.</ref> |
| DeltaHf = −276 kJ/mol | | DeltaHf = −276 kJ/mol | ||
| DeltaHc = −1367.3 kJ/mol | | DeltaHc = −1367.3 kJ/mol | ||
| Line 61: | Line 61: | ||
== History == | == History == | ||
| − | Ethanol has been used by humans since prehistory as the intoxicating ingredient in [[alcoholic beverage]]s. Dried residues on 9000-year-old pottery found in northern [[mainland China]] imply the use of alcoholic beverages even among [[Neolithic]] peoples.<ref name="Roach">{{citation | last = Roach | first = J. |date = 2005-07-18 | url = http://news.nationalgeographic.com/news/2005/07/0718_050718_ancientbeer.html | title = 9,000-Year-Old Beer Re-Created From Chinese Recipe | work = National Geographic News | online = yes | accessdate = 2005-11-14}}.</ref> Its isolation as a relatively pure compound was first achieved by [[Persian people|Persian]] alchemists who developed the art of [[distillation]] during the [[Abbasid caliphate]], the most notable of whom was [[Al-Razi]]. The writings attributed to [[Jabir Ibn Hayyan]] (Geber) (721–815) mention the flammable vapors of boiled wine. [[Al-Kindī]] (801–873) unambiguously described the distillation of wine.<ref name="al-Hassan">{{citation | + | Ethanol has been used by humans since prehistory as the intoxicating ingredient in [[alcoholic beverage]]s. Dried residues on 9000-year-old pottery found in northern [[mainland China]] imply the use of alcoholic beverages even among [[Neolithic]] peoples.<ref name="Roach">{{citation | last = Roach | first = J. |date = 2005-07-18 | url = http://news.nationalgeographic.com/news/2005/07/0718_050718_ancientbeer.html | title = 9,000-Year-Old Beer Re-Created From Chinese Recipe | work = National Geographic News | online = yes | accessdate = 2005-11-14}}.</ref> Its isolation as a relatively pure compound was first achieved by [[Persian people|Persian]] alchemists who developed the art of [[distillation]] during the [[Abbasid caliphate]], the most notable of whom was [[Al-Razi]]. The writings attributed to [[Jabir Ibn Hayyan]] (Geber) (721–815) mention the flammable vapors of boiled wine. [[Al-Kindī]] (801–873) unambiguously described the distillation of wine.<ref name="al-Hassan">{{citation | first = Ahmad Y. | last = Hassan | url = http://www.gabarin.com/ayh/Notes/Notes%207.htm | title = Alcohol and the Distillation of Wine in Arabic Sources | online = yes | accessdate = 2005-11-14}}.</ref> Distillation of ethanol from [[water]] yields a product that is at most 95.6% ethanol, because ethanol forms an [[azeotrope]] with water. Absolute ethanol was first obtained in 1796 by Johann Tobias Lowitz, by filtering distilled ethanol through [[Activated carbon|charcoal]]. |
| − | [[Antoine Lavoisier]] described ethanol as a compound of carbon, hydrogen, and oxygen, | + | [[Antoine Lavoisier]] described ethanol as a compound of carbon, hydrogen, and oxygen and, in 1808, [[Nicolas-Théodore de Saussure]] determined ethanol's chemical formula,<ref>{{citation | contribution = Alcohol | year = 1911 | editor-first = Hugh | editor-last = Chisholm | title = Encyclopædia Britannica | edition = 11th | url = http://91.1911encyclopedia.org/A/AL/ALCOHOL.htm}}.</ref> and fifty years later, in 1858, [[Archibald Scott Couper]] published a structural formula for ethanol:<ref name="Couper">{{citation | last = Couper | first = A. S. | year = 1858 | title = On a new chemical theory | url = http://web.lemoyne.edu/~giunta/couper/couper.html | journal = Phil. Mag. | volume = 16 | pages = 104–16}}.</ref> this places ethanol among the first chemical compounds to have their chemical structures determined. |
Ethanol was first prepared synthetically in 1826, through the independent efforts of Henry Hennel in Great Britain and S.G. Sérullas in [[France]]. [[Michael Faraday]] prepared ethanol by the [[Acid catalysis|acid-catalysed]] hydration of [[ethylene]] in 1828, in a process similar to that used for industrial ethanol synthesis today.<ref name="Hennell">{{citation | last = Hennell | first = H. | year = 1828 | title = On the mutual action of sulfuric acid and alcohol, and on the nature of the process by which ether is formed | journal = Phil. Trans. | volume = 118 | pages = 365–71}}.</ref> | Ethanol was first prepared synthetically in 1826, through the independent efforts of Henry Hennel in Great Britain and S.G. Sérullas in [[France]]. [[Michael Faraday]] prepared ethanol by the [[Acid catalysis|acid-catalysed]] hydration of [[ethylene]] in 1828, in a process similar to that used for industrial ethanol synthesis today.<ref name="Hennell">{{citation | last = Hennell | first = H. | year = 1828 | title = On the mutual action of sulfuric acid and alcohol, and on the nature of the process by which ether is formed | journal = Phil. Trans. | volume = 118 | pages = 365–71}}.</ref> | ||
| Line 124: | Line 124: | ||
:C<sub>2</sub>H<sub>4</sub> + H<sub>2</sub>O → CH<sub>3</sub>CH<sub>2</sub>OH | :C<sub>2</sub>H<sub>4</sub> + H<sub>2</sub>O → CH<sub>3</sub>CH<sub>2</sub>OH | ||
| − | The catalyst is most commonly [[phosphoric acid]], [[adsorption|adsorbed]] onto a porous support such as [[diatomaceous earth]] or [[charcoal]]; this catalyst was first used for large-scale ethanol production by the [[Shell Oil Company]] in 1947.<ref name="ECT4">{{ | + | The catalyst is most commonly [[phosphoric acid]], [[adsorption|adsorbed]] onto a porous support such as [[diatomaceous earth]] or [[charcoal]]; this catalyst was first used for large-scale ethanol production by the [[Shell Oil Company]] in 1947.<ref name="ECT4">{{Kirk-Othmer4th | last = Lodgsdon | first = J. E. | contribution = Ethanol | volume = 9 | page = 812–60}}.</ref> Solid catalysts, mostly various metal oxides, have also been mentioned in the chemical literature. |
In an older process, first practiced on the industrial scale in 1930 by [[Union Carbide]],<ref name="ECT4"/> but now almost entirely obsolete, ethene was hydrated indirectly by reacting it with concentrated [[sulfuric acid]] to product [[ethyl sulfate]], which was then [[hydrolysis|hydrolysed]] to yield ethanol and regenerate the sulfuric acid: | In an older process, first practiced on the industrial scale in 1930 by [[Union Carbide]],<ref name="ECT4"/> but now almost entirely obsolete, ethene was hydrated indirectly by reacting it with concentrated [[sulfuric acid]] to product [[ethyl sulfate]], which was then [[hydrolysis|hydrolysed]] to yield ethanol and regenerate the sulfuric acid: | ||
| Line 232: | Line 232: | ||
Ethanol is easily [[soluble]] in [[water (molecule)|water]] in all proportions with a slight overall decrease in volume when the two are mixed. Absolute ethanol and 95% ethanol are themselves good [[solvent]]s, somewhat less polar than water and used in [[perfume]]s, [[paint]]s and [[tincture]]s. Other proportions of ethanol with water or other solvents can also be used as a solvent. Alcoholic drinks have a large variety of tastes because various flavor compounds are dissolved during [[brewing]]. When ethanol is produced as a mixing beverage it is a [[neutral grain spirit]]. | Ethanol is easily [[soluble]] in [[water (molecule)|water]] in all proportions with a slight overall decrease in volume when the two are mixed. Absolute ethanol and 95% ethanol are themselves good [[solvent]]s, somewhat less polar than water and used in [[perfume]]s, [[paint]]s and [[tincture]]s. Other proportions of ethanol with water or other solvents can also be used as a solvent. Alcoholic drinks have a large variety of tastes because various flavor compounds are dissolved during [[brewing]]. When ethanol is produced as a mixing beverage it is a [[neutral grain spirit]]. | ||
| − | Ethanol is used in medical wipes and in most common antibacterial hand sanitizer gels at a concentration of about 62% ([[percentage]] by weight, not volume) as an [[antiseptic]]. The peak of the disinfecting power occurs around 70% ethanol; stronger and weaker solutions of ethanol have a lessened ability to disinfect. Solutions of this strength are often used in laboratories for disinfecting work surfaces. Ethanol kills organisms by denaturing their [[protein]]s and dissolving their [[lipid]]s and is effective against most [[bacterium|bacteria]] and [[fungus|fungi]], and many [[virus]]es, but is ineffective against bacterial [[spore]]s. Alcohol does not act like an [[antibiotic]] and is not effective against [[infection]]s by ingestion. Ethanol in the low concentrations typically found in most alcoholic beverages does not have useful disinfectant or antiseptic properties, internally or externally. Ethanol is often used as an antidote in cases of methanol poisoning. | + | Ethanol is used in medical wipes and in most common antibacterial hand sanitizer gels at a concentration of about 62% ([[percentage]] by weight, not volume) as an [[antiseptic]]. The peak of the disinfecting power occurs around 70% ethanol; stronger and weaker solutions of ethanol have a lessened ability to disinfect.<ref>{{citation | title = Guidelines for Hand Hygiene in Healthcare Settings | url = http://www.cdc.gov/mmwr/PDF/rr/rr5116.pdf | series = Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force | publisher = Centers for Disease Control and Prevention | location = Atlanta, Ga. | journal = Morbid. Mort. Weekly Rep. | year = 2002 | volume = 51 | issue = RR-16}}.</ref> Solutions of this strength are often used in laboratories for disinfecting work surfaces. Ethanol kills organisms by denaturing their [[protein]]s and dissolving their [[lipid]]s and is effective against most [[bacterium|bacteria]] and [[fungus|fungi]], and many [[virus]]es, but is ineffective against bacterial [[spore]]s. Alcohol does not act like an [[antibiotic]] and is not effective against [[infection]]s by ingestion. Ethanol in the low concentrations typically found in most alcoholic beverages does not have useful disinfectant or antiseptic properties, internally or externally. Ethanol is often used as an antidote in cases of methanol poisoning. |
Wine with less than 16% ethanol cannot protect itself against bacteria. Because of this, [[port wine|port]] is often fortified with ethanol to at least 18% ethanol by volume to halt [[fermentation (food)|fermentation]] for retaining sweetness and in preparation for aging, at which point it becomes possible to prevent the invasion of bacteria into the port, and to store the port for long periods of time in wooden containers that can 'breathe', thereby permitting the port to age safely without spoiling. Because of ethanol's disinfectant property, alcoholic beverages of 18% ethanol or more by volume can be safely stored for a very long time. | Wine with less than 16% ethanol cannot protect itself against bacteria. Because of this, [[port wine|port]] is often fortified with ethanol to at least 18% ethanol by volume to halt [[fermentation (food)|fermentation]] for retaining sweetness and in preparation for aging, at which point it becomes possible to prevent the invasion of bacteria into the port, and to store the port for long periods of time in wooden containers that can 'breathe', thereby permitting the port to age safely without spoiling. Because of ethanol's disinfectant property, alcoholic beverages of 18% ethanol or more by volume can be safely stored for a very long time. | ||
| Line 264: | Line 264: | ||
== See also == | == See also == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* [[Biodiesel]] | * [[Biodiesel]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* [[Rubbing alcohol]] | * [[Rubbing alcohol]] | ||
| − | |||
| − | == References == | + | ==References== |
| − | {{reflist | + | {{reflist|2}} |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== External links == | == External links == | ||
| − | *{{ICSC| | + | *{{ICSC|0044}} |
*{{PGCH|0262}} | *{{PGCH|0262}} | ||
| − | * | + | *{{NPI|id=35|name=Ethanol (ethyl alcohol)}} |
*[http://www.ethanol-information.com/ Ethanol Information] | *[http://www.ethanol-information.com/ Ethanol Information] | ||
| − | *[http://www.compchemwiki.org/index.php?title=Ethanol Coordinates of the ethanol molecule] on Computational Chemistry Wiki | + | *[http://www.compchemwiki.org/index.php?title=Ethanol Coordinates of the ethanol molecule] on Computational Chemistry Wiki |
*[http://www.bluerhinos.co.uk/molview/indv.php?id=4 Molview from bluerhinos.co.uk] See Ethanol in 3D | *[http://www.bluerhinos.co.uk/molview/indv.php?id=4 Molview from bluerhinos.co.uk] See Ethanol in 3D | ||
| − | |||
*[http://www.distill.com/specs/US-2.html Specifications] | *[http://www.distill.com/specs/US-2.html Specifications] | ||
| − | *[http://www.unitedbioenergy.com United Bio Energy | + | *[http://www.unitedbioenergy.com United Bio Energy (UBE)] General information on ethanol plants and products. Also industry links. |
| − | + | *[http://sci-toys.com/ingredients/alcohol.html Sci-toys website explanation of US denatured alcohol designations] | |
| − | |||
[[Category:Alcohols]] | [[Category:Alcohols]] | ||
{{Imported from Wikipedia|name=Ethanol|id=102133699}} | {{Imported from Wikipedia|name=Ethanol|id=102133699}} | ||
Latest revision as of 08:01, 1 September 2009
| Ethanol | |
|---|---|
| IUPAC name | Ethanol |
| Other names | Ethyl alcohol; grain alcohol; pure alcohol; hydroxyethane; drinking alcohol; ethyl hydrate; absolute alcohol |
| Identifiers | |
| InChI | InChI=1/C2H6O/c1-2-3/h3H,2H2,1H3 |
| InChIKey | LFQSCWFLJHTTHZ-UHFFFAOYAB |
| Standard InChI | InChI=1S/C2H6O/c1-2-3/h3H,2H2,1H3 |
| Standard InChIKey | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| CAS number | [] |
| EC number | |
| RTECS | KQ6300000 |
| ChemSpider | |
| PubChem | |
| SMILES | |
| Properties[1][2] | |
| Molecular formula | C2H6O |
| Molar mass | 46.07 g mol−1 |
| Appearance | colorless liquid |
| Density | 0.789 g/cm3 |
| Melting point |
−114.3 ºC (158.8 K) |
| Boiling point |
78.4 ºC (351.5 K) |
| Triple point | 150±20 K |
| Critical point | 514±7 K, 63±4 bar |
| Solubility in water | miscible |
| log P | −0.32 |
| Acidity (pKa) | 15.9 |
| Refractive index (nD) | 1.3614 |
| Viscosity | 1.200 cP (20 °C) |
| Dipole moment | 1.69 D (gas) |
| Thermochemistry[2][3] | |
| Std enthalpy of formation ΔfH |
−276 kJ/mol |
| Std enthalpy of combustion ΔcH |
−1367.3 kJ/mol |
| Standard molar entropy S |
160.6 J K−1 mol−1 |
| Std enthalpy of vaporization ΔvapH |
38.6 kJ/mol |
| Hazards[1][4][5] | |
| EU index number | 603-002-00-5 |
| GHS pictograms | 
|
| GHS signal word | DANGER |
| GHS hazard statements | H225 |
| Flash point | 13 °C (55 °F) |
| Autoignition temp. | 363 ºC (685 ºF) |
| Explosive limits | 3.3–19% |
| PEL (U.S.) | 1000 ppm TWA |
| Related compounds | |
| Other alcohols | Methanol Propanol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
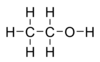
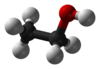
Ethanol, also known as ethyl alcohol or grain alcohol, is a flammable, colorless, slightly toxic chemical compound with a distinctive perfume-like odor, and is the alcohol found in alcoholic beverages. In common usage, it is often referred to simply as alcohol. Its molecular formula is variously represented as EtOH, CH3CH2OH, C2H5OH or as its empirical formula C2H6O.
Contents
History
Ethanol has been used by humans since prehistory as the intoxicating ingredient in alcoholic beverages. Dried residues on 9000-year-old pottery found in northern mainland China imply the use of alcoholic beverages even among Neolithic peoples.[6] Its isolation as a relatively pure compound was first achieved by Persian alchemists who developed the art of distillation during the Abbasid caliphate, the most notable of whom was Al-Razi. The writings attributed to Jabir Ibn Hayyan (Geber) (721–815) mention the flammable vapors of boiled wine. Al-Kindī (801–873) unambiguously described the distillation of wine.[7] Distillation of ethanol from water yields a product that is at most 95.6% ethanol, because ethanol forms an azeotrope with water. Absolute ethanol was first obtained in 1796 by Johann Tobias Lowitz, by filtering distilled ethanol through charcoal.
Antoine Lavoisier described ethanol as a compound of carbon, hydrogen, and oxygen and, in 1808, Nicolas-Théodore de Saussure determined ethanol's chemical formula,[8] and fifty years later, in 1858, Archibald Scott Couper published a structural formula for ethanol:[9] this places ethanol among the first chemical compounds to have their chemical structures determined.
Ethanol was first prepared synthetically in 1826, through the independent efforts of Henry Hennel in Great Britain and S.G. Sérullas in France. Michael Faraday prepared ethanol by the acid-catalysed hydration of ethylene in 1828, in a process similar to that used for industrial ethanol synthesis today.[10]
Ethanol fuel has been important in the past, and as petroleum increases in price, it is becoming increasingly important again in this role.
Physical properties
Ethanol's hydroxyl group is able to participate in hydrogen bonding. At the molecular level, liquid ethanol consists of hydrogen-bonded pairs of ethanol molecules; this phenomenon renders ethanol more viscous and less volatile than less polar organic compounds of similar molecular weight. In the vapor phase, there is little hydrogen bonding; ethanol vapor consists of individual ethanol molecules. Ethanol, like most short-chain alcohols, is flammable, colorless, has a strong odor, and is volatile.
Ethanol is a versatile solvent. It is miscible with water and with most organic liquids, including nonpolar liquids such as aliphatic hydrocarbons. Organic solids of low molecular weight are usually soluble in ethanol. Among ionic compounds, many monovalent salts are at least somewhat soluble in ethanol, with salts of large, polarizable ions being more soluble than salts of smaller ions. Most salts of polyvalent ions are practically insoluble in ethanol.
Furthermore, ethanol is used as a solvent in dissolving medicines, food flavourings and colourings that do not dissolve easily in water. Once the non-polar material is dissolved in the ethanol, water can be added to prepare a solution that is mostly water. The ethanol molecule has a hydrophilic –OH group that helps it dissolve polar molecules and ionic substances. The short, hydrophobic hydrocarbon chain CH3CH2– can attract non-polar molecules. Thus ethanol can dissolve both polar and non-polar substances.
Several unusual phenomena are associated with mixtures of ethanol and water. Ethanol-water mixtures have less volume than their individual components: a mixture of equal volumes ethanol and water has only 95.6% of the volume of equal parts ethanol and water, unmixed. The addition of even a few percent of ethanol to water sharply reduces the surface tension of water. This property partially explains the tears of wine phenomenon: when wine is swirled inside a glass, ethanol evaporates quickly from the thin film of wine on the wall of the glass. As its ethanol content decreases, its surface tension increases, and the thin film beads up and runs down the glass in channels rather than as a smooth sheet.
Chemistry
The chemistry of ethanol is largely that of its hydroxyl group.
- Acid-base chemistry
Ethanol's hydroxyl proton is very weakly acidic; it is an even weaker acid than water. Ethanol can be quantitatively converted to its conjugate base, the ethoxide ion (CH3CH2O−), by reaction with an alkali metal such as sodium. This reaction evolves hydrogen gas:
- 2CH3CH2OH + 2Na → 2CH3CH2ONa + H2
- Nucleophilic substitution
In aprotic solvents, ethanol reacts with hydrogen halides to produce ethyl halides such as ethyl chloride and ethyl bromide via nucleophilic substitution:
- CH3CH2OH + HCl → CH3CH2Cl + H2O
- CH3CH2OH + HBr → CH3CH2Br + H2O
Ethyl halides can also be produced by reacting ethanol by more specialized halogenating agents, such as thionyl chloride for preparing ethyl chloride, or phosphorus tribromide for preparing ethyl bromide.
- Esterification
Under acid-catalysed conditions, ethanol reacts with carboxylic acids to produce ethyl esters and water:
- RCOOH + HOCH2CH3 → RCOOCH2CH3 + H2O
The reverse reaction, hydrolysis of the resulting ester back to ethanol and the carboxylic acid, limits the extent of reaction, and high yields are unusual unless water can be removed from the reaction mixture as it is formed. Esterification can also be carried out using more a reactive derivative of the carboxylic acid, such as an acyl chloride or acid anhydride. A very common ester of ethanol is ethyl acetate, found in for example nailpolish remover.
Ethanol can also form esters with inorganic acids. Diethyl sulfate and triethyl phosphate, prepared by reacting ethanol with sulfuric and phosphoric acid, respectively, are both useful ethylating agents in organic synthesis. Ethyl nitrite, prepared from the reaction of ethanol with sodium nitrite and sulfuric acid, was formerly a widely-used diuretic.
- Dehydration
Strong acids, such as sulfuric acid, can catalyse the dehydration of ethanol to form either diethyl ether or ethylene:
- 2CH3CH2OH → CH3CH2OCH2CH3 + H2O
- CH3CH2OH → H2C=CH2 + H2O
Although sulfuric acid catalyses this reaction, the acid is diluted by the water that is formed, which makes the reaction inefficient. Which product predominates, diethyl ether or ethylene, depends on the precise reaction conditions.
- Oxidation
Ethanol can be oxidized to acetaldehyde, and further oxidized to acetic acid. In the human body, these oxidation reactions are catalysed by enzymes. In the laboratory, aqueous solutions of strong oxidizing agents, such as chromic acid or potassium permanganate, oxidize ethanol to acetic acid, and it is difficult to stop the reaction at acetaldehyde at high yield. Ethanol can be oxidized to acetaldehyde, without overoxidation to acetic acid, by reacting it with pyridinium chromic chloride.
- Combustion
Combustion of ethanol forms carbon dioxide and water:
- C2H5OH + 3O2 → 2CO2 +3H2O
Production
Ethanol is produced both as a petrochemical, through the hydration of ethylene, and biologically, by fermenting sugars with yeast.
Ethylene hydration
Ethanol for use as industrial feedstock is most often made from petrochemical feedstocks, typically by the acid-catalyzed hydration of ethene, represented by the chemical equation
- C2H4 + H2O → CH3CH2OH
The catalyst is most commonly phosphoric acid, adsorbed onto a porous support such as diatomaceous earth or charcoal; this catalyst was first used for large-scale ethanol production by the Shell Oil Company in 1947.[11] Solid catalysts, mostly various metal oxides, have also been mentioned in the chemical literature.
In an older process, first practiced on the industrial scale in 1930 by Union Carbide,[11] but now almost entirely obsolete, ethene was hydrated indirectly by reacting it with concentrated sulfuric acid to product ethyl sulfate, which was then hydrolysed to yield ethanol and regenerate the sulfuric acid:
- C2H4 + H2SO4 → CH3CH2OSO3H
- CH3CH2SO4H + H2O → CH3CH2OH + H2SO4
Fermentation
Ethanol for use in alcoholic beverages, and the vast majority of ethanol for use as fuel, is produced by fermentation: when certain species of yeast (most importantly, Saccharomyces cerevisiae) metabolize sugar in the absence of oxygen, they produce ethanol and carbon dioxide. The overall chemical reaction conducted by the yeast may be represented by the chemical equation
- C6H12O6 → 2CH3CH2OH + 2CO2
The process of culturing yeast under conditions to produce alcohol is referred to as brewing. Brewing can only produce relatively dilute concentrations of ethanol in water; concentrated ethanol solutions are toxic to yeast. The most ethanol-tolerant strains of yeast can survive in up to about 15% ethanol (by volume).
During the fermentation process, it is important to prevent oxygen getting to the ethanol, since otherwise the ethanol would be oxidised to acetic acid (vinegar). Also, in the presence of oxygen, the yeast would undergo aerobic respiration to produce just carbon dioxide and water, without producing ethanol.
In order to produce ethanol from starchy materials such as cereal grains, the starch must first be broken down into sugars. In brewing beer, this has traditionally been accomplished allowing the grain to germinate, or malt. In the process of germination, the seed produces enzymes that can break its starches into sugars. For fuel ethanol, this hydrolysis of starch into glucose is accomplished more rapidly by treatment with dilute sulfuric acid, fungal amylase enzymes, or some combination of the two.
At petroleum prices like those that prevailed through much of the 1990s, ethylene hydration was a decidedly more economical process than fermentation for producing purified ethanol. Recent increases in petroleum prices, coupled with perennial uncertainty in agricultural prices, make forecasting the relative production costs of fermented versus petrochemical ethanol difficult at the present time.
Purification

The product of either ethylene hydration or brewing is an ethanol-water mixture. For most industrial and fuel uses, the ethanol must be purified. Fractional distillation can concentrate ethanol to 95.6% volume; the mixture of 95.6% ethanol and 4.4% water (percentage by weight) is an azeotrope with a boiling point of 78.2 °C, and cannot be further purified by distillation. Therefore, 95% ethanol in water is a fairly common solvent.
After distillation ethanol can be further purified by "drying" it using lime or salt. Lime, (calcium oxide), when mixed with the water in ethanol will form calcium hydroxide, which then can be separated. Dry salt will dissolve some of the water content of the ethanol as it passes through, leaving a purer alcohol.[12]
Several approaches are used to produce absolute ethanol. The ethanol-water azeotrope can be broken by the addition of a small quantity of benzene. Benzene, ethanol, and water form a ternary azeotrope with a boiling point of 64.9 °C. Since this azeotrope is more volatile than the ethanol-water azeotrope, it can be fractionally distilled out of the ethanol-water mixture, extracting essentially all of the water in the process. The bottoms from such a distillation is anhydrous ethanol, with several parts per million residual benzene. Benzene is toxic to humans, and cyclohexane has largely supplanted benzene in its role as the entrainer in this process.
Alternatively, a molecular sieve can be used to selectively absorb the water from the 95.6% ethanol solution. Synthetic zeolite in pellet form can be used, as well as a variety of plant-derived absorbents, including cornmeal, straw, and sawdust. The zeolite bed can be regenerated essentially an unlimited number of times by drying it with a blast of hot carbon dioxide. Cornmeal and other plant-derived absorbents cannot readily be regenerated, but where ethanol is made from grain, they are often available at low cost. Absolute ethanol produced this way has no residual benzene, and can be used to fortify port and sherry in traditional winery operations. Pervaporation and Vapor Permeation are membrane processes that breaks the water ethanol azeotrope and does not need regeneration.
At pressures less than atmospheric pressure, the composition of the ethanol-water azeotrope shifts to more ethanol-rich mixtures, and at pressures less than 70 torr (9.333 kPa) , there is no azeotrope, and it is possible to distill absolute ethanol from an ethanol-water mixture. While vacuum distillation of ethanol is not presently economical, pressure-swing distillation is a topic of current research. In this technique, a reduced-pressure distillation first yields an ethanol-water mixture of more than 95.6% ethanol. Then, fractional distillation of this mixture at atmospheric pressure distills off the 95.6% azeotrope, leaving anhydrous ethanol at the bottoms.
Prospective technologies
Glucose for fermentation into ethanol can also be obtained from cellulose. Until recently, however, the cost of the cellulase enzymes that could hydrolyse cellulose has been prohibitive. The Canadian firm Iogen brought the first cellulose-based ethanol plant on-stream in 2004.[13] The primary consumer thus far has been the Canadian government, which, along with the United States government (particularly the Department of Energy's National Renewable Energy Laboratory), has invested millions of dollars into assisting the commercialization of cellulosic ethanol. Realization of this technology would turn a number of cellulose-containing agricultural byproducts, such as corncobs, straw, and sawdust, into renewable energy resources.
Other enzyme companies are developing genetically engineered fungi which would produce large volumes of cellulase, xylanase and hemicellulase enzymes which can be utilized to convert agricultural residues such as corn stover, distiller grains, wheat straw and sugar cane bagasse and energy crops such as Switchgrass into fermentable sugars which may be used to produce cellulosic ethanol.Template:Cn
Cellulosic materials typically contain, in addition to cellulose, other polysaccharides, including hemicellulose. When hydrolysed, hemicellulose breaks down into mostly five-carbon sugars such as xylose. S. cerevisiae, the yeast most commonly used for ethanol production, cannot metabolize xylose. Other yeasts and bacteria are under investigation to metabolize xylose and so improve the ethanol yield from cellulosic material.[14][15]
The anaerobic bacterium Clostridium ljungdahlii, recently discovered in commercial chicken wastes, can produce ethanol from single-carbon sources including synthesis gas, a mixture of carbon monoxide and hydrogen that can be generated from the partial combustion of either fossil fuels or biomass. Use of these bacteria to produce ethanol from synthesis gas has progressed to the pilot plant stage at the BRI Energy facility in Fayetteville, Arkansas.[16]
Another prospective technology is the closed-loop ethanol plant. Ethanol produced from corn has a number of critics who suggest that it is primarily just recycled fossil fuels because of the energy required to grow the grain and convert it into ethanol. However, the closed-loop ethanol plant attempts to address this criticism. In a closed-loop plant, the energy for the distillation comes from fermented manure, produced from cattle that have been fed the by-products from the distillation. The leftover manure is then used to fertilize the soil used to grow the grain. Such a process is expected to have a much lower fossil fuel requirement. However, general thermodynamic considerations indicate that the total efficiency of such plants, in combination with the production of cellulose/sugar, will remain relatively low.
Types of ethanol
Denatured alcohol
In most jurisdictions, the sale of ethanol, as a pure substance, or in the form of alcoholic beverages, is heavily taxed. In order to relieve non-beverage industries of this tax burden, governments specify formulations for denatured alcohol, which consists of ethanol blended with various additives to render it unfit for human consumption. These additives, called denaturants, are generally either toxic (such as methanol) or have unpleasant tastes or odors (such as denatonium benzoate).
Specialty denatured alcohols are denatured alcohol formulations intended for a particular industrial use, containing denaturants chosen so as not to interfere with that use. While they are not taxed, purchasers of specialty denatured alcohols must have a government-issued permit for the particular formulation they use and must comply with other regulations.
Completely denatured alcohols are formulations that can be purchased for any legal purpose, without permit, bond, or other regulatory compliance. It is intended that it be difficult to isolate a product fit for human consumption from completely denatured alcohol. For example, the completely denatured alcohol formulation used in the United Kingdom contains (by volume) 89.66% ethanol, 9.46% methanol, 0.50% pyridine, 0.38% naphtha, and is dyed purple with methyl violet.[17]
Anhydrous Alcohol can also be produced from hydrous(95-96%) alcohol using drying agents like molecular sieves, or by azeotropic distillation, extractive distillation techniques.
Absolute ethanol
Absolute or anhydrous alcohol generally refers to purified ethanol, containing no more than one percent water.
It is not possible to obtain absolute alcohol by simple fractional distillation, because a mixture containing around 95.6% alcohol and 4.4% water becomes a constant boiling mixture (an azeotropic mixture). In one common industrial method to obtain absolute alcohol, a small quantity of benzene is added to rectified spirit and the mixture is then distilled. Absolute alcohol is obtained in third fraction that distills over at 78.2 ºC (351.3 K). Because a small amount of the benzene used remains in the solution, absolute alcohol produced by this method is not suitable for consumption as benzene is carcinogenic.
There is also an absolute alcohol production process by desiccation using glycerol. Alcohol produced by this method is known as spectroscopic alcohol - so called because the absence of benzene makes it suitable as a solvent in spectroscopy.
Currently, the most popular method of purification past 95.6% purity is desiccation using adsorbents such as starch or zeolites, which adsorb water preferentially,. Azeotropic distillation and extractive distillation techniques also exist.
Feedstocks
Currently the main feedstock in the United States for the production of ethanol is corn (see the Renewable Fuels Association's list of U.S. ethanol for a complete list and feedstock utilized. Approximately 2.8 gallons of ethanol are produced from one bushel of corn. While much of the corn turns into ethanol, some of the corn also yields by-products such as DDGS (distillers dried grains with solubles) that can be used to fullfill a portion of the diet of livestock. A bushel of corn produces about 18 pounds of DDGS[18]). Trials of a new crop, switchgrass, are showing much greater yields.[ref. needed] The dominant ethanol feedstock in warmer regions is sugarcane. In some parts of Europe, particularly France and Italy, wine is used as a feedstock due to massive oversupply.
Use
As a fuel
The largest single use of ethanol is as a motor fuel and fuel additive. The largest national fuel ethanol industries exist in Brazil (gasoline sold in Brazil contains at least 20% ethanol and hydrous ethanol is also used as fuel).[19] One method of production is through fermentation of sugar. Ethanol creates very little pollution when burned. Millions more acres of land are needed if ethanol is to be used to replace gasoline. Pure ethanol has a lower energy content than gasoline (about 30% less energy per unit volume).[20] At gas stations, ethanol is contained in a mix of ethanol and gasoline, otherwise known as gasohol. In the United States, the color yellow (symbolizing the color of corn) has become associated with the fuel and is commonly used on fuel pumps and labels.
According to the Renewable Fuels Association, as of November 2006; 107 grain ethanol biorefineries in the United States have the capacity to produce 5.1 billion gallons of ethanol per year. An additional 56 construction projects underway (in the U.S.) can add 3.8 billion gallons of new capacity in the next 18 months. Over time, it is believed that a material portion of the roughly 150 billion gallon per year market for gasoline will begin to be replaced with fuel ethanol.[21] Growth in fuel ethanol in the United States is largely being driven by financial incentives that naturally exist when oil prices are over a certain level, as ethanol typically costs under $1.50 per gallon to manufacture (of course this is sensitive to corn prices) and is exempt from the $0.52 per gallon federal gasoline tax. However, the United States RFS (renewable fuel standard) requires that 4 billion gallons of "renewable fuel" be used in 2006 and this requirement will grow to 7.5 billion gallons per annum by 2012.[22]
Alcoholic beverages
Alcoholic beverages vary considerably in their ethanol content and in the foodstuffs from which they are produced. Most alcoholic beverages can be broadly classified as fermented beverages, beverages made by the action of yeast on sugary foodstuffs, or as distilled beverages, beverages whose preparation involves concentrating the ethanol in fermented beverages by distillation. The ethanol content of a beverage is usually measured in terms of the volume fraction of ethanol in the beverage, expressed either as a percentage or in alcoholic proof units.
Fermented beverages can be broadly classified by the foodstuff from which they are fermented. Beers are made from cereal grains or other starchy materials, wines and ciders from fruit juices, and meads from honey. Cultures around the world have made fermented beverages from numerous other foodstuffs, and local and national names for various fermented beverages abound. Fermented beverages may contain up to 15–20% ethanol by volume, the upper limit being set by the yeast's tolerance for ethanol, or by the amount of sugar in the starting material.
Distilled beverages are made by distilling fermented beverages. Broad categories of distilled beverages include whiskies, distilled from fermented cereal grains; brandies, distilled from fermented fruit juices, and rum, distilled from fermented molasses or sugarcane juice. Vodka and similar neutral grain spirits can be distilled from any fermented material (grain or potatoes is most common); these spirits are so thoroughly distilled that no tastes from the particular starting material remain. Numerous other spirits and liqueurs are prepared by infusing flavors from fruits, herbs, and spices into distilled spirits. A traditional example is gin, the infusion of juniper berries into neutral grain alcohol.
In a few beverages, ethanol is concentrated by means other than distillation. Applejack is traditionally made by freeze distillation: water is frozen out of fermented apple cider, leaving a more ethanol-rich liquid behind. Fortified wines are prepared by adding brandy or some other distilled spirit to partially-fermented wine. This kills the yeast and conserves some of the sugar in grape juice; such beverages are not only more ethanol-rich, but are often sweeter than other wines.
Chemicals derived from ethanol
- Ethyl esters
In the presence of an acid catalyst (typically sulfuric acid) ethanol reacts with carboxylic acids to produce ethyl esters:
- CH3CH2OH + RCOOH → RCOOCH2CH3 + H2O
The two largest-volume ethyl esters are ethyl acrylate (from ethanol and acrylic acid) and ethyl acetate (from ethanol and acetic acid). Ethyl acrylate is a monomer used to prepare acrylate polymers for use in coatings and adhesives. Ethyl acetate is a common solvent used in paints, coatings, and in the pharmaceutical industry; its most familiar application in the household is as a solvent for nail polish. A variety of other ethyl esters are used in much smaller volumes as artificial fruit flavorings.
- Vinegar
Vinegar is a dilute solution of acetic acid prepared by the action of Acetobacter bacteria on ethanol solutions. Although traditionally prepared from alcoholic beverages including wine, apple cider, and unhopped beer, vinegar can also be made from solutions of industrial ethanol. Vinegar made from distilled ethanol is called "distilled vinegar", and is commonly used in food pickling and as a condiment.
- Ethylamines
When heated to 150–220 °C over a silica- or alumina-supported nickel catalyst, ethanol and ammonia react to produce ethylamine. Further reaction leads to diethylamine and triethylamine:
- CH3CH2OH + NH3 → CH3CH2NH2 + H2O
- CH3CH2OH + CH3CH2NH2 → (CH3CH2)2NH + H2O
- CH3CH2OH + (CH3CH2)2NH → (CH3CH2)3N + H2O
The ethylamines find use in the synthesis of pharmaceuticals, agricultural chemicals, and surfactants.
- Other chemicals
Ethanol in the past has been used commercially to synthesize dozens of other high-volume chemical commodities. At the present, it has been supplanted in many applications by less costly petrochemical feedstocks. However, in markets with abundant agricultural products, but a less developed petrochemical infrastructure, such as the People's Republic of China, Pakistan, India, and Brazil, ethanol can be used to produce chemicals that would be produced from petroleum in the West, including ethylene and butadiene.
Other uses
Ethanol is easily soluble in water in all proportions with a slight overall decrease in volume when the two are mixed. Absolute ethanol and 95% ethanol are themselves good solvents, somewhat less polar than water and used in perfumes, paints and tinctures. Other proportions of ethanol with water or other solvents can also be used as a solvent. Alcoholic drinks have a large variety of tastes because various flavor compounds are dissolved during brewing. When ethanol is produced as a mixing beverage it is a neutral grain spirit.
Ethanol is used in medical wipes and in most common antibacterial hand sanitizer gels at a concentration of about 62% (percentage by weight, not volume) as an antiseptic. The peak of the disinfecting power occurs around 70% ethanol; stronger and weaker solutions of ethanol have a lessened ability to disinfect.[23] Solutions of this strength are often used in laboratories for disinfecting work surfaces. Ethanol kills organisms by denaturing their proteins and dissolving their lipids and is effective against most bacteria and fungi, and many viruses, but is ineffective against bacterial spores. Alcohol does not act like an antibiotic and is not effective against infections by ingestion. Ethanol in the low concentrations typically found in most alcoholic beverages does not have useful disinfectant or antiseptic properties, internally or externally. Ethanol is often used as an antidote in cases of methanol poisoning.
Wine with less than 16% ethanol cannot protect itself against bacteria. Because of this, port is often fortified with ethanol to at least 18% ethanol by volume to halt fermentation for retaining sweetness and in preparation for aging, at which point it becomes possible to prevent the invasion of bacteria into the port, and to store the port for long periods of time in wooden containers that can 'breathe', thereby permitting the port to age safely without spoiling. Because of ethanol's disinfectant property, alcoholic beverages of 18% ethanol or more by volume can be safely stored for a very long time.
Metabolism and toxicology
Pure ethanol is a tasteless liquid with a strong and distinctive odor that produces a characteristic heat-like sensation when brought into contact with the tongue or mucous membranes. When applied to open wounds (as for disinfection) it produces a strong stinging sensation. Pure or highly concentrated ethanol may permanently damage living tissue on contact. Ethanol applied to unbroken skin cools the skin rapidly through evaporation.
In the human body, ethanol is first oxidized to acetaldehyde, and then to acetic acid. The first step is catalysed by the enzyme alcohol dehydrogenase, and the second by acetaldehyde dehydrogenase. Some individuals have less effective forms of one or both of these enzymes, and can experience more severe symptoms from ethanol consumption than others. Conversely, those who have acquired ethanol tolerance have a greater quantity of these enzymes, and metabolize ethanol more rapidly.
| BAC (mg/dL) | Symptoms |
|---|---|
| 50 | Euphoria, talkativeness, relaxation |
| 100 | Central nervous system depression, impaired motor and sensory function, impaired cognition |
| >140 | Decreased blood flow to brain |
| 300 | Stupefaction, possible unconsciousness |
| 400 | Possible death |
| >550 | Death highly likely |
The amount of ethanol in the body is typically quantified by blood alcohol content (BAC), the milligrams of ethanol per 100 milliliters of blood. The table at right summarizes the symptoms of ethanol consumption. Small doses of ethanol generally produce euphoria and relaxation; people experiencing these symptoms tend to become talkative and less inhibited, and may exhibit poor judgment. At higher dosages (BAC > 0.10), ethanol acts as a central nervous system depressant, producing at progressively higher dosages, impaired sensory and motor function, slowed cognition, stupefaction, unconsciousness, and possible death.
The initial product of ethanol metabolism, acetaldehyde, is more toxic than ethanol itself. The body can quickly detoxify some acetaldehyde by reaction with glutathione and similar thiol-containing biomolecules. When acetaldehyde is produced beyond the capacity of the body's glutathione supply to detoxify it, it accumulates in the bloodstream until further oxidized to acetic acid. The headache, nausea, and malaise associated with an alcohol hangover stem from a combination of dehydration and acetaldehyde poisoning; many health conditions associated with chronic ethanol abuse, including liver cirrhosis, alcoholism, and some forms of cancer, have been linked to acetaldehyde.[ref. needed] The judicial system in the United States, in a number of jurisdictions, promoted the use of disulfiram, known as Antabuse, for persons convicted of driving while (alcohol) intoxicated. Disulfuram interferes with hepatic acetaldehyde metabolism, exacerbating the discomforts noted above. Numerous deaths, said to be related to disulfuram use, led to the elimination of these court-based programs. Some medications, including paracetamol (acetaminophen), as well as exposure to organochlorides, can deplete the body's glutathione supply, enhancing both the acute and long-term risks of even moderate ethanol consumption. Frequent use of alcoholic beverages has also been shown to be a major contributing factor in cases of elevated blood levels of triglycerides.[25]
Hazards
- Ethanol-water solutions greater than about 50% ethanol by volume are flammable and easily ignited. Ethanol-water solutions below 50% ethanol by volume may also be flammable if the solution is vaporized by heating (as in some cooking methods that call for wine to be added to a hot pan, causing it to flash boil into a vapor, which is then ignited to "burn off" excessive alcohol).
See also
References
- ↑ 1.0 1.1 Ethanol (anhydrous); International Chemical Safety Card 0044; International Labour Organization: Geneva, October 2000, <http://www.inchem.org/documents/icsc/icsc/eics0044.htm>.
- ↑ 2.0 2.1 Ethyl alcohol. In NIST Chemistry WebBook; National Institute for Standards and Technology, <http://webbook.nist.gov/cgi/inchi/InChI%3D1S/C2H6O/c1-2-3/h3H,2H2,1H3>. (accessed 21 August 2009).
- ↑ Kelley, Kenneth K. The heat capacities of ethyl and hexyl alcohols from 16°K to 298°K and the corresponding entropies and free energies. J. Am. Chem. Soc. 1929, 51 (3), 779–86. DOI: 10.1021/ja01378a016. Green, J. H. S. Revision of the values of the heats of formation of normal alcohols. Chem. Ind. (London) 1960, 1215–16. Green, J. H. S. Thermodynamic properties of organic oxygen compounds. Part 5. Ethyl alcohol. Trans. Faraday Soc. 1961, 57, 2132–37. DOI: 10.1039/TF9615702132. Chao, Jing; Rossini, F. D. Heats of combustion, formation, and isomerization of nineteen alkanols. J. Chem. Eng. Data 1965, 10 (4), 374–79. DOI: 10.1021/je60027a022. Haida, Osamu; Suga, Hiroshi; Seki, Syûzô Calorimetric study of the glassy state. XII. Plural glass-transition phenomena of ethanol. J. Chem. Thermodynam. 1977, 9 (12), 1133–48. DOI: 10.1016/0021-9614(77)90115-X. Majer, Vladimír; Svoboda, Václav Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation; Blackwell: Oxford, 1985. ISBN 0632015292.
- ↑ Index no. 603-002-00-5 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 477.
- ↑ Ethyl alcohol. In Pocket Guide to Chemical Hazards; U.S. Department of Health and Human Services (NIOSH) Publication No. 2005-149; Government Printing Office: Washington, DC, 2005. ISBN 9780160727511, <http://www.cdc.gov/niosh/npg/npgd0262.html>.
- ↑ Roach, J. 9,000-Year-Old Beer Re-Created From Chinese Recipe. National Geographic News 2005-07-18, <http://news.nationalgeographic.com/news/2005/07/0718_050718_ancientbeer.html>. (accessed 14 November 2005).
- ↑ Hassan, Ahmad Y. Alcohol and the Distillation of Wine in Arabic Sources, <http://www.gabarin.com/ayh/Notes/Notes%207.htm>. (accessed 14 November 2005).
- ↑ Alcohol. In Encyclopædia Britannica, 11th ed.; Chisholm, Hugh, Ed., 1911, <http://91.1911encyclopedia.org/A/AL/ALCOHOL.htm>.
- ↑ Couper, A. S. On a new chemical theory. Phil. Mag. 1858, 16, 104–16, <http://web.lemoyne.edu/~giunta/couper/couper.html>.
- ↑ Hennell, H. On the mutual action of sulfuric acid and alcohol, and on the nature of the process by which ether is formed. Phil. Trans. 1828, 118, 365–71.
- ↑ 11.0 11.1 Lodgsdon, J. E. Ethanol. In Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; Kroschwitz, J. I.; Howe-Grant, M., Eds.; John Wiley: New York, 1994; Vol. 9, p 812–60.
- ↑ Mathewson, S. W. Drying the Alcohol. In The Manual for the Home and Farm Production of Alcohol Fuel; Ten Speed Press, 1980, <http://www.journeytoforever.org/biofuel_library/ethanol_manual/manual12.html>. (accessed 1 July 2006)
- ↑ Ritter, S. K. Biomass or Bust. Chem. Eng. News 2004-05-31, 82 (22), 31–34.
- ↑ , <http://www.lub.lu.se/cgi-bin/show_diss.pl?db=global&fname=tec_748.html>
- ↑ , <http://www.metabolicengineering.gov/me2001/2001Kompala.pdf>
- ↑ , <http://www.brienergy.com/>
- ↑ Denatured Alcohol Regulations 2005 No. 1524.
- ↑ , <http://www.ethanolrfa.org/industry/locations/>.
- ↑ Reel, M. Brazil's Road to Energy Independence. Washington Post 2006-08-19, <http://www.washingtonpost.com/wp-dyn/content/article/2006/08/19/AR2006081900842.html>.
- ↑ Fueltable, <http://www.eere.energy.gov/afdc/pdfs/fueltable.pdf>.
- ↑ , <http://www.ethanolrfa.org/media/press/rfa/view.php?id=909>.
- ↑ , <http://www.epa.gov/otaq/renewablefuels/>.
- ↑ Guidelines for Hand Hygiene in Healthcare Settings. Morbid. Mort. Weekly Rep., Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force (Centers for Disease Control and Prevention: Atlanta, Ga.) 2002, 51 (RR-16), <http://www.cdc.gov/mmwr/PDF/rr/rr5116.pdf>.
- ↑ Pohorecky, L. A.; Brick, J. Pharmacology of ethanol. Pharmacol. Ther. 1988, 36 (3), 335–427. PMID 3279433.
- ↑ , <http://www.americanheart.org/presenter.jhtml?identifier=4778>.
External links
- International Chemical Safety Card 0044
- NIOSH Pocket Guide to Chemical Hazards 0262
- Entry for "Ethanol (ethyl alcohol)" on the Australian National Pollutant Inventory
- Ethanol Information
- Coordinates of the ethanol molecule on Computational Chemistry Wiki
- Molview from bluerhinos.co.uk See Ethanol in 3D
- Specifications
- United Bio Energy (UBE) General information on ethanol plants and products. Also industry links.
- Sci-toys website explanation of US denatured alcohol designations
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Ethanol". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |