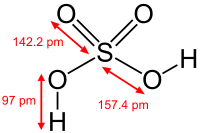
Sulfuric acid
| Sulfuric acid | |
|---|---|
| |

| |
| IUPAC name | Sulfuric acid |
| Other names | Oil of vitriol |
| Identifiers | |
| InChI | InChI=1/H2O4S/c1-5(2,3)4/h(H2,1,2,3,4) |
| InChIKey | QAOWNCQODCNURD-UHFFFAOYAC |
| Standard InChI | InChI=1S/H2O4S/c1-5(2,3)4/h(H2,1,2,3,4) |
| Standard InChIKey | QAOWNCQODCNURD-UHFFFAOYSA-N |
| CAS number | [] |
| EC number | |
| UN number | 1830 |
| RTECS | WS5600000 |
| ChemSpider | |
| Properties[1] | |
| Chemical formula | H2SO4 |
| Molar mass | 98.079 g/mol |
| Appearance | colorless, oily liquid |
| Density | 1.8267 g/cm3 (25 ºC), liquid |
| Melting point |
10.371 ºC (283.521 K) |
| Boiling point |
approx. 300 ºC (573 K) decomp. |
| Solubility in water | miscible |
| Acidity (pKa) | −3 |
| Viscosity | 24.55 cP (25 °C) |
| Hazards[2][3] | |
| Material safety data sheet (MSDS) | ICSC |
| EU index number | 016-020-00-8 |
| GHS pictograms | 
|
| GHS signal word | DANGER |
| GHS hazard statements | H314 |
| Flash point | non-flammable |
| PEL (U.S.) | 1 ppm TWA |
| IDLH level | 15 ppm |
| Related compounds | |
| Other strong acids | Selenic acid Hydrochloric acid Nitric acid |
| Other compounds | Sulfurous acid Peroxymonosulfuric acid Sulfur trioxide Oleum |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Sulfuric (or sulphuric) acid, H2SO4, is a strong mineral acid. It is soluble in water at all concentrations. Sulfuric acid has many applications, and is one of the top products of the chemical industry. World production in 2001 was 165 million tonnes, with an approximate value of US$8 billion. Principal uses include lead-acid batteries for cars and other vehicles, ore processing, fertilizer manufacturing, oil refining, wastewater processing, and chemical synthesis.
Contents
Occurrence
Pure (undiluted) sulfuric acid is not encountered naturally on Earth, due to its great affinity for water. Apart from that, sulfuric acid is a constituent of acid rain, which is formed by atmospheric oxidation of sulfur dioxide in the presence of water. Sulfur dioxide is the main byproduct produced when sulfur-containing fuels such as coal or oil are burned.
Sulfuric acid is formed naturally by the oxidation of sulfide minerals, such as iron sulfide. The resulting water can be highly acidic and is called acid mine drainage (AMD) or acid rock drainage (ARD). This acidic water is capable of dissolving metals present in sulfide ores, which results in brightly-colored, toxic streams. The oxidation of iron sulfide pyrite by molecular oxygen produces iron(II), or Fe2+:
- 2FeS2 + 7O2 + 2H2O → 2Fe2+ + 4SO42− + 4H+
The Fe2+ can be further oxidized to Fe3+:
- 4Fe2+ + O2 + 4H+ → 4Fe3+ + 2 H2O
The Fe3+ produced can be precipitated as the hydroxide or hydrous oxide:
- Fe3+ + 3H2O → Fe(OH)3 + 3H+
The iron(III) ion ("ferric iron") can also oxidize pyrite. When iron(III) oxidation of pyrite occurs, the process can become rapid. ARD produced by this process can have pH values below zero.
ARD can also produce sulfuric acid at a slower rate, so that the Acid Neutralization Capacity (ANC) of the aquifer can neutralize the produced acid. In such cases, the total dissolved solids (TDS) concentration of the water can be increased from the dissolution of minerals from the acid-neutralization reaction with the minerals.
Extraterrestrial sulfuric acid
Atmosphere of Venus
Sulfuric acid is produced in the upper atmosphere of Venus by the Sun's photochemical action on carbon dioxide, sulfur dioxide, and water vapor. Ultraviolet photons of wavelengths less than 169 nm can photodissociate carbon dioxide into carbon monoxide and atomic oxygen.
Atomic oxygen is highly reactive. When it reacts with sulfur dioxide, a trace component of the Venusian atmosphere, the result is sulfur trioxide, which can combine with water vapor, another trace component of Venus's atmosphere, to yield sulfuric acid.
- CO2 → CO + O
- SO2 + O → SO3
- SO3 + H2O → H2SO4
In the upper, cooler portions of Venus's atmosphere, sulfuric acid exists as a liquid, and thick sulfuric acid clouds completely obscure the planet's surface when viewed from above. The main cloud layer extends from 45–70 km above the planet's surface, with thinner hazes extending as low as 30 km and as high as 90 km above the surface.
The permanent Venusian clouds produce a concentrated acid rain, as the clouds in the atmosphere of Earth produce water rain.
The atmosphere exhibits a sulfuric acid cycle. As sulfuric acid rain droplets fall down through the hotter layers of the atmosphere's temperature gradient, they are heated up and release water vapor, becoming more and more concentrated. When they reach temperatures above 300 °C, sulfuric acid begins to decompose into sulfur trioxide and water, both in the gas phase. Sulfur trioxide is highly reactive and dissociates into sulfur dioxide and atomic oxygen, which oxidizes traces of carbon monoxide to form carbon dioxide.
Sulfur dioxide and water vapor rise on convection currents from the mid-level atmospheric layers to higher altitudes, where they will be transformed again into sulfuric acid, and the cycle repeats.
On the surface of Europa
Infrared spectra from NASA's Galileo mission show distinct absorptions on Jupiter's moon Europa that have been attributed to one or more sulfuric acid hydrates. The interpretation of the spectra is somewhat controversial. Some planetary scientists prefer to assign the spectral features to the sulfate ion, perhaps as part of one or more minerals on Europa's surface.[4]
Manufacture
Sulfuric acid is produced from sulfur, oxygen and water via the conventional contact process (DCDA) or the wet sulfuric acid process (WSA).
- Description of the Contact process (DCDA)
In the first step, sulfur is burned to produce sulfur dioxide.
- S + O2 → SO2
This is then oxidized to sulfur trioxide using oxygen in the presence of a vanadium(V) oxide catalyst.
- 2SO2 + O2 → 2SO3 (in presence of V2O5)
The sulfur trioxide is absorbed into 97–98% H2SO4 to form oleum (H2S2O7), also known as fuming sulfuric acid. The oleum is then diluted with water to form concentrated sulfuric acid.
- H2SO4 + SO3 → H2S2O7
- H2S2O7 + H2O → 2 H2SO4
Note that directly dissolving SO3 in water is not practical due to the highly exothermic nature of the reaction between sulfur trioxide and water. The reaction forms a corrosive aerosol that is very difficult to separate, instead of a liquid.
- SO3 + H2O → H2SO4
- Description of the Wet sulfuric acid process (WSA)
In the first step, either sulfur is burned to produce sulfur dioxide.
- S + O2 → SO2
or hydrogen sulfide is incinerated to SO2 gas.
- H2S + 3⁄2O2 → H2O + SO2
This is then oxidized to sulfur trioxide using oxygen in the presence of a vanadium(V) oxide catalyst.
- 2SO2 + O2 → 2SO3 (in presence of V2O5)
The sulfur trioxide is hydrated in the gas phase to sulfuric acid:
- SO3 + H2O → H2SO4
The last step is the condensation of the sulfuric acid to liquid 97–98% H2SO4
- H2SO4(g) + 0.17H2O(g) → H2SO4(l)
Prior to 1900, most sulfuric acid was manufactured by the chamber process.[5]
Physical properties
Forms of sulfuric acid
Although nearly 100% sulfuric acid can be made, this loses SO3 at the boiling point to produce 98.3% acid. The 98% grade is more stable in storage, and is the usual form of what is described as "concentrated sulfuric acid". Other concentrations are used for different purposes. Some common concentrations are
- 10%, "dilute sulfuric acid" for laboratory use;
- 33.53%, "battery acid" (used in lead–acid batteries),
- 62.18%, "chamber acid" or "fertilizer acid";
- 73.61%, "tower acid" or "Glover acid".
Different purities are also available. Technical grade H2SO4 is impure and often colored, but is suitable for making fertilizer. Pure grades such as United States Pharmacopoeia (USP) grade are used for making pharmaceuticals and dyestuffs.
When high concentrations of SO3 are added to sulfuric acid, H2S2O7, called pyrosulfuric acid, fuming sulfuric acid or oleum or, less commonly, Nordhausen acid, is formed. Concentrations of oleum are either expressed in terms of percentage SO3 (called %oleum) or as percentage H2SO4 (the amount made if H2O were added); common concentrations are 40% oleum (109% H2SO4) and 65% oleum (114.6% H2SO4). Pure H2S2O7 is a solid with melting point 36 °C.
Polarity and conductivity
Anhydrous H2SO4 is a very polar liquid, having a dielectric constant of around 100. It has a high electrical conductivity, caused by dissociation through protonating itself, a process known as autoprotolysis.[1]
- 2H2SO4 ⇌ H3SO4+ + HSO4−
- K (25 °C) = [H3SO4+][HSO4−] = 2.7 × 10−4.
The comparable equilibrium constant for water, Kw is 10−14, a factor of 1010 (10 billion) smaller.
In spite of the viscosity of the acid, the effective conductivities of the H3SO4+ and HSO4− ions are high due to an intramolecular proton-switch mechanism (analogous to the Grotthuss mechanism in water), making sulfuric acid a good conductor. It is also an excellent solvent for many reactions.
The equilibrium is actually more complex than shown above; 100% H2SO4 contains the following species at equilibrium (figures shown as millimol per kg solvent): HSO4− (15.0), H3SO4+ (11.3), H3O+ (8.0), HS2O7− (4.4), H2S2O7 (3.6), H2O (0.1).[1]
Chemical properties
Reaction with water
The hydration reaction of sulfuric acid is highly exothermic (ΔrH ≈ −880 kJ/mol). One should always add the acid to the water rather than the water to the acid, because of the relative densities of these two liquids. Water is less dense than sulfuric acid, and will tend to float on top of it. Thus, if water is added to the concentrated sulfuric acid, it can boil and splatter dangerously.
Because the hydration of sulfuric acid is thermodynamically favorable, sulfuric acid is an excellent dehydrating agent, and is used to prepare many dried fruits. The affinity of sulfuric acid for water is sufficiently strong that it will remove hydrogen and oxygen atoms from other compounds; for example, mixing starch (C6H12O6)n and concentrated sulfuric acid will give elemental carbon and water which is absorbed by the sulfuric acid (which becomes slightly diluted):
- (C6H12O6)n → 6nC + 6nH2O
The effect of this can be seen when concentrated sulfuric acid is spilled on paper; the cellulose reacts to give a burnt appearance, the carbon appears much as soot would in a fire. A more dramatic reaction occurs when sulfuric acid is added to a tablespoon of white sugar; a rigid column of black, porous carbon will quickly emerge. The carbon will smell strongly of caramel.
Other reactions
As an acid, sulfuric acid reacts with most bases to give the corresponding sulfate. For example, the blue copper salt copper(II) sulfate, commonly used for electroplating and as a fungicide, can be prepared by the reaction of copper(II) oxide with sulfuric acid:
- CuO + H2SO4 → CuSO4 + H2O
Sulfuric acid can also be used to displace weaker acids from their salts. Reaction with sodium acetate, for example, displaces acetic acid, CH3COOH, and forms sodium bisulfate:
- H2SO4 + CH3COONa → NaHSO4 + CH3COOH
Similarly, reacting sulfuric acid with potassium nitrate can be used to produce nitric acid and a precipitate of potassium bisulfate. When combined with nitric acid, sulfuric acid acts both as an acid and a dehydrating agent, forming the nitronium ion NO2+, which is important in nitration reactions involving electrophilic aromatic substitution. This type of reaction, where protonation occurs on an oxygen atom, is important in many organic chemistry reactions, such as Fischer esterification and dehydration of alcohols.
Sulfuric acid reacts with most metals via a single displacement reaction to produce hydrogen gas and the metal sulfate. Dilute H2SO4 attacks iron, aluminium, zinc, manganese, magnesium and nickel, but reactions with tin and copper require the acid to be hot and concentrated. Lead and tungsten, however, are resistant to sulfuric acid. The reaction with iron shown below is typical for most of these metals, but the reaction with tin produces sulfur dioxide rather than hydrogen.
- Fe + H2SO4 → H2 + FeSO4
- Sn + 2H2SO4 → SnSO4 + 2H2O + SO2
These reactions may be taken as typical: the hot concentrated acid generally acts as an oxidising agent whereas the dilute acid acts a typical acid. Hence hot concentrated acid reacts with tin, zinc and copper to produce the salt, water and sulphur dioxide, whereas the dilute acid reacts with metals high in the reactivity series (such as zinc) to produce a salt and hydrogen.[6]
Sulfuric acid undergoes electrophilic aromatic substitution with aromatic compounds to give the corresponding sulfonic acids:[7]
Uses
Sulfuric acid is a very important commodity chemical, and indeed, a nation's sulfuric acid production is a good indicator of its industrial strength.[8] The major use (60% of total production worldwide) for sulfuric acid is in the "wet method" for the production of phosphoric acid, used for manufacture of phosphate fertilizers as well as trisodium phosphate for detergents. In this method, phosphate rock is used, and more than 100 million tonnes are processed annually. This raw material is shown below as fluorapatite, though the exact composition may vary. This is treated with 93% sulfuric acid to produce calcium sulfate, hydrogen fluoride (HF) and phosphoric acid. The HF is removed as hydrofluoric acid. The overall process can be represented as:
- Ca5F(PO4)3 + 5H2SO4 + 10H2O → 5CaSO4·2H2O + HF + 3H3PO4
Sulfuric acid is used in large quantities by the iron and steelmaking industry to remove oxidation, rust and scale from rolled sheet and billets prior to sale to the automobile and white-goods industry. Used acid is often recycled using a Spent Acid Regeneration (SAR) plant. These plants combust spent acid with natural gas, refinery gas, fuel oil or other fuel sources. This combustion process produces gaseous sulfur dioxide (SO2) and sulfur trioxide (SO3) which are then used to manufacture "new" sulfuric acid. SAR plants are common additions to metal smelting plants, oil refineries, and other industries where sulfuric acid is consumed in bulk, as operating a SAR plant is much cheaper than the recurring costs of spent acid disposal and new acid purchases.
Ammonium sulfate, an important nitrogen fertilizer, is most commonly produced as a byproduct from coking plants supplying the iron and steel making plants. Reacting the ammonia produced in the thermal decomposition of coal with waste sulfuric acid allows the ammonia to be crystallized out as a salt (often brown because of iron contamination) and sold into the agro-chemicals industry.
Another important use for sulfuric acid is for the manufacture of aluminum sulfate, also known as papermaker's alum. This can react with small amounts of soap on paper pulp fibers to give gelatinous aluminum carboxylates, which help to coagulate the pulp fibers into a hard paper surface. It is also used for making aluminum hydroxide, which is used at water treatment plants to filter out impurities, as well as to improve the taste of the water. Aluminum sulfate is made by reacting bauxite with sulfuric acid:
- Al2O3 + 3H2SO4 → Al2(SO4)3 + 3H2O
Sulfuric acid is used for a variety of other purposes in the chemical industry. For example, it is the usual acid catalyst for the conversion of cyclohexanoneoxime to caprolactam, used for making nylon. It is used for making hydrochloric acid from salt via the Mannheim process. Much H2SO4 is used in petroleum refining, for example as a catalyst for the reaction of isobutane with isobutylene to give isooctane, a compound that raises the octane rating of gasoline (petrol). Sulfuric acid is also important in the manufacture of dyestuffs solutions and is the "acid" in lead-acid (car) batteries.
Sulfuric acid is also used as a general dehydrating agent in its concentrated form. See Reaction with water.
Sulfur–iodine cycle
The sulfur–iodine cycle is a series of thermo-chemical processes used to obtain hydrogen. It consists of three chemical reactions whose net reactant is water and whose net products are hydrogen and oxygen.
2H2SO4 → 2SO2 + 2H2O + O2 (830 °C) I2 + SO2 + 2H2O → 2HI + H2SO4 (120 °C) 2HI → I2 + H2 (320 °C)
The sulfur and iodine compounds are recovered and reused, hence the consideration of the process as a cycle. This process is endothermic and must occur at high temperatures, so energy in the form of heat has to be supplied.
The sulfur–iodine cycle has been proposed as a way to supply hydrogen for a hydrogen-based economy. It does not require hydrocarbons like current methods of steam reforming, but the concentrated, corrosive acid at high temperatures poses currently insurmountable safety hazards if the process were built on a large-scale.
History

The discovery of sulfuric acid is credited to the 8th century Muslim chemist and alchemist, Jabir ibn Hayyan (Geber). The acid was later studied by 9th century Persian physician and alchemist Ibn Zakariya al-Razi (Rhazes), who obtained the substance by dry distillation of minerals including iron(II) sulfate heptahydrate, FeSO4·7H2O, and copper(II) sulfate pentahydrate, CuSO4·5H2O. When heated, these compounds decompose to iron(II) oxide and copper(II) oxide, respectively, giving off water and sulfur trioxide, which combine to produce a dilute solution of sulfuric acid. This method was popularized in Europe through translations of Arabic and Persian treatises, as well as books by European alchemists, such as the 13th-century German Albertus Magnus.
Sulfuric acid was known to medieval European alchemists as oil of vitriol, spirit of vitriol, or simply vitriol, among other names. The word vitriol derives from the Latin vitreus, "glass", referring to the glassy appearance of the hydrated sulfate salts, which also carried the name vitriol. Salts called by this name included copper(II) sulfate (blue vitriol, or rarely Roman vitriol), zinc sulfate (white vitriol), iron(II) sulfate (green vitriol), iron(III) sulfate (vitriol of Mars), and cobalt(II) sulfate (red vitriol). Red Vitriol is also a 20th century technical name for a grade of sulphuric acid.
Vitriol was widely considered the most important alchemical substance, intended to be used as a philosopher's stone. Highly purified vitriol was used as a medium for reacting other substances. This was largely because the acid does not react with gold, production of which was often the final goal of alchemical processes. The importance of vitriol to alchemy is highlighted in the alchemical motto, Visita Interiora Terrae Rectificando Invenies Occultum Lapidem which is a backronym meaning ('Visit the interior of the earth and rectifying (i.e. purifying) you will find the hidden/secret stone'), found in L'Azoth des Philosophes by the 15th century alchemist Basilius Valentinus, .
In the 17th century, the German-Dutch chemist Johann Glauber prepared sulfuric acid by burning sulfur together with saltpeter (potassium nitrate, KNO3), in the presence of steam. As saltpeter decomposes, it oxidizes the sulfur to SO3, which combines with water to produce sulfuric acid. In 1736, Joshua Ward, a London pharmacist, used this method to begin the first large-scale production of sulfuric acid.
In 1746 in Birmingham, John Roebuck adapted this method to produce sulfuric acid in lead-lined chambers, which were stronger, less expensive, and could be made larger than the previously used glass containers. This lead chamber process allowed the effective industrialization of sulfuric acid production. After several refinements, this method remained the standard for sulfuric acid production for almost two centuries.
Sulfuric acid created by John Roebuck's process only approached a 35–40% concentration.[ref. needed] Later refinements to the lead-chamber process by French chemist Joseph-Louis Gay-Lussac and British chemist John Glover yielded 78% acid.[ref. needed] However, the manufacture of some dyes and other chemical processes require a more concentrated product. Throughout the 18th century, this could only be made by dry distilling minerals in a technique similar to the original alchemical processes. Pyrite (iron disulfide, FeS2) was heated in air to yield iron (II) sulfate, FeSO4, which was oxidized by further heating in air to form iron(III) sulfate, Fe2(SO4)3, which, when heated to 480 °C, decomposed to iron(III) oxide and sulfur trioxide, which could be passed through water to yield sulfuric acid in any concentration. However, the expense of this process prevented the large-scale use of concentrated sulfuric acid.
In 1831, British vinegar merchant Peregrine Phillips patented the contact process, which was a far more economical process for producing sulfur trioxide and concentrated sulfuric acid. Today, nearly all of the world's sulfuric acid is produced using this method.
Safety
Laboratory hazards
The corrosive properties of sulfuric acid are accentuated by its highly exothermic reaction with water. Burns from sulfuric acid are potentially more serious than those of comparable strong acids (e.g. hydrochloric acid), as there is additional tissue damage due to dehydration and particularly secondary thermal damage due to the heat liberated by the reaction with water.
The danger is greater with more concentrated preparations of sulfuric acid, but even the normal laboratory "dilute" grade (approximately 1 M, 10%) will char paper by dehydration if left in contact for a sufficient time. Therefore, solutions equal to or stronger than 1.5 M are labeled "CORROSIVE", while solutions greater than 0.5 M but less than 1.5 M are labeled "IRRITANT". Fuming sulfuric acid (oleum) is not recommended for use in schools due to it being quite hazardous.
The standard first aid treatment for acid spills on the skin is, as for other corrosive agents, irrigation with large quantities of water. Washing is continued for at least ten to fifteen minutes to cool the tissue surrounding the acid burn and to prevent secondary damage. Contaminated clothing is removed immediately and the underlying skin washed thoroughly.
Preparation of the diluted acid can also be dangerous due to the heat released in the dilution process. The concentrated acid is always added to water and not the other way round, to take advantage of the relatively high heat capacity of water. Addition of water to concentrated sulfuric acid leads to the dispersal of a sulfuric acid aerosol or worse, an explosion. Preparation of solutions greater than 6 M (35%) in concentration is most dangerous, as the heat produced may be sufficient to boil the diluted acid: efficient mechanical stirring and external cooling (such as an ice bath) are essential.
On a laboratory scale, sulfuric acid is advantageously diluted by pouring the concentrated version onto crushed ice. The ice used is sufficiently chemically pure so as not to interfere with the intended use of the diluted acid.
Industrial hazards
Although sulfuric acid is non-flammable, contact with metals in the event of a spillage can lead to the liberation of hydrogen gas. The dispersal of acid aerosols and gaseous sulfur dioxide is an additional hazard of fires involving sulfuric acid.
Sulfuric acid is not considered toxic besides its obvious corrosive hazard, and the main occupational risks are skin contact leading to burns (see above) and the inhalation of aerosols. Exposure to aerosols at high concentrations leads to immediate and severe irritation of the eyes, respiratory tract and mucous membranes: this ceases rapidly after exposure, although there is a risk of subsequent pulmonary edema if tissue damage has been more severe. At lower concentrations, the most commonly reported symptom of chronic exposure to sulfuric acid aerosols is erosion of the teeth, found in virtually all studies: indications of possible chronic damage to the respiratory tract are inconclusive as of 1997. Interestingly there have been reports of sulfuric acid ingestion leading to vitamin B12 deficiency with subacute combined degeneration. The spinal cord is most often affected in such cases, but the optic nerves may show demyelination, loss of axons and gliosis.
Legal restrictions
International commerce of sulfuric acid is controlled under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances, 1988, which lists sulfuric acid under Table II of the convention as a chemical frequently used in the illicit manufacture of narcotic drugs or psychotropic substances.[9]
In the United States, sulfuric acid is included in List II of the list of essential or precursor chemicals established pursuant to the Chemical Diversion and Trafficking Act. Accordingly, transactions of sulfuric acid—such as sales, transfers, exports from and imports to the United States—are subject to regulation and monitoring by the Drug Enforcement Administration.
References
- ↑ 1.0 1.1 1.2 Greenwood, Norman N.; Earnshaw, A. Chemistry of the Elements; Pergamon: Oxford, 1984; pp 837–45. ISBN 0-08-022057-6.
- ↑ Index no. 016-020-00-8 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 400.
- ↑ Sulfuric acid. In Pocket Guide to Chemical Hazards; U.S. Department of Health and Human Services (NIOSH) Publication No. 2005-149; Government Printing Office: Washington, DC, 2005. ISBN 9780160727511, <http://www.cdc.gov/niosh/npg/npgd0577.html>.
- ↑ Orlando, Thomas M.; McCord, Thomas B.; Grieves, Gregory A. The chemical nature of Europa surface material and the relation to a subsurface ocean. Icarus 2005, 177 (2), 528–33. DOI: 10.1016/j.icarus.2005.05.009.
- ↑ Jones, Edward M. Chamber Process Manufacture of Sulfuric Acid. Ind. Eng. Chem. 1950 (11), 2208–10. DOI: 10.1021/ie50491a016.
- ↑ Holderness, A.; Lambert, John A New Certificate Chemistry, 5th ed.; Heinemann: London, 1977. ISBN 0435644246.
- ↑ Carey, F. A. Reactions of Arenes. Electrophilic Aromatic Substitution, <http://www.chem.ucalgary.ca/courses/351/Carey/Ch12/ch12-4.html> (accessed 27 January 2008), On-Line Learning Center for Organic Chemistry; University of Calgary.
- ↑ Chenier, Philip J. Survey of Industrial Chemistry; Wiley: New York, 1986; pp 45–57. ISBN 0471010774.
- ↑ List of Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances under International Control, 11th ed.; International Narcotics Control Board: Vienna, 2007, <http://www.incb.org/pdf/e/list/red.pdf>.
Further reading
- Acide sulfurique; Fiche toxicologique n° 30; Institut National de Recherche et de Sécurité: Paris, 1997.
- CRC Handbook of Chemistry and Physics, 71st ed.; CRC Press: Ann Arbor, MI, 1990.
- Agamanolis, D. P. Metabolic and toxic disorders. In Neuropathology; Prayson, Richard A., Ed.; Foundations in Diagnostic Pathology series; Churchill Livingstone: Philadelphia, 2005; pp 413–15. ISBN 0443066582.
External links
- International Chemical Safety Card 0362
- NIOSH Pocket Guide to Chemical Hazards 0577
- Sulfuric acid analysis - titration freeware
- - Sulfuric Acid density and pH-value at t=20°C
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Sulfuric acid". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |